15. State - Wildlife
Le rapport sur l’état de l’environnement 2022 est un document technique destiné à un usage interne. Il n’est disponible qu’en anglais.
Introduction
The Northwest Territories is one of the few remaining regions in the world where large tracts of landscape with complex northern-adapted ecosystems exist with intact food chains and rich northern biodiversity. Wildlife is one of the main links between people, cultures, and the environment. The indicators presented in this focal point reveal that changes are occurring in wildlife of the NWT.

Caribou are a major focal species, and one of the indicators provides information on all caribou ecotypes and sub-species present in the NWT. Another indicator provides information on changes and declines for birds of particular importance to the people of the NWT. Two indicators present information on wildlife health, one in terrestrial and one in aquatic environments. Other factors influencing wildlife health are presented in the Contaminants focal point. Trends in the number of new species in the NWT, as well as species that are expanding their ranges to new areas within the NWT are tracked as such changes are predicted to occur with a warming climate.
15.1 Trends in caribou
This indicator measures the trend in population sizes of caribou (Rangifer tarandus) in the NWT. It includes all subspecies and ecotypes of caribou present in the NWT including Peary caribou (R. t. pearyi), Dolphin and Union caribou (R. t. pearyi x groenlandicus), barren-ground caribou and Porcupine caribou (R. t. groenlandicus), boreal caribou (R. t. caribou; boreal woodland ecotype), and northern mountain caribou (R. t. caribou; northern mountain woodland ecotype).

This indicator merges information from indicators used in previous reports 15.3 (Trend in barren-ground caribou population size in tundra-taiga ecosystems), 16.3. (Status of Peary caribou in a changing climate), and 16.6. (Status of woodland caribou in a changing landscape).
This indicator was prepared by the Government of the Northwest Territories, Department of Environment and Climate Change, using information obtained from various sources including from surveys conducted by wildlife management agencies in collaboration with wildlife co-management partners. Surveys for most NWT herds and populations are conducted by the Government of the Northwest Territories with input from Indigenous governments, Indigenous organizations, renewable resources boards and other co-management partners. Surveys for some shared herds and populations are done by NWT communities, Aboriginal Governments, and by neighbouring wildlife agencies in Nunavut, Yukon, British Columbia, Alberta, and Alaska.
This indicator merges information from indicators used in previous reports 15.3 (Trend in barren-ground caribou population size in tundra-taiga ecosystems), 16.3. (Status of Peary caribou in a changing climate), and 16.6. (Status of woodland caribou in a changing landscape).
NWT Focus
Caribou are an important source of food, clothing, and cultural identity for Indigenous people of the NWT. Caribou are one of the NWT’s most important wildlife resources.
Some of the factors that affect caribou cannot be directly controlled (e.g., weather and climate) but management actions can be taken to address the impacts of human activities such as hunting and development. When populations are declining or are at low numbers, they are less resilient to changes to their environment and climate. Status and trend information allows wildlife co-management partners to identify which management actions are most appropriate to ensure that caribou populations remain a healthy and resilient component of our northern ecosystems.
Current View: status and trend
Peary Caribou
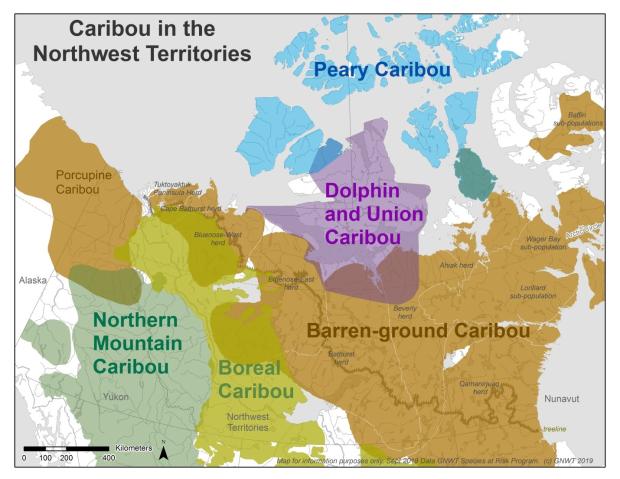
Peary caribou are small light coloured caribou that live in the harsh treeless environment of the Arctic Islands. Peary caribou exist only in Canada (Figure 1). About 7000-8000 Peary Caribou, or nearly 60% of the Canadian population, are found in the NWT (Ref. 1, Ref. 2).
Peary caribou numbers have sometimes declined rapidly in the past, especially during winter rain on snow events or other harsh climatic events (Figure 2). To obtain food in the winter, caribou must dig or paw down to the vegetation under the snow. This is easier in areas where wind has removed most of the snow, and in years when less snow is present. Rain in the fall and winter can create ground-fast ice that restricts the ability of the animals to reach forage.

There is evidence that Peary caribou populations can increase when there are favourable environmental conditions, including periods with less frequent periods of deep snow and ice. Peary caribou are known to move between arctic islands in search of better habitat, which has led to sub-populations being identified that include groups of islands. The impact of harsh winters on Peary caribou on the High Arctic islands (Borden, Mackenzie King and Brock Islands) is unknown. However, results from the 2012 surveys on the western Queen Elizabeth Islands were encouraging as more Peary caribou were seen and some areas were occupied that were not in the previous decade (Ref. 3). Signs of recovery followed by die-offs on Prince Patrick and Melville Islands suggest that it is likely that large weather events can affect Peary caribou populations on several High Arctic Islands (Ref. 4).
Banks Island has relatively lush vegetation compared to most High Arctic Islands and its climate is milder. However, as on islands further north, unusually mild and wet winters, especially with freezing rain, have resulted in die-offs and lower calf production of Peary caribou in some years. A combination of factors may explain the low recovery of Peary caribou numbers on Banks Island, including competition with muskoxen for food or feeding areas, increased predation pressure, and movement of caribou off the island (Ref. 5).
There is little weather data available for Banks Island to track the effects of mild deep-snow winters and rain on snow events on large herbivores on the island (Ref. 6, Ref 7). Caribou hunting on Banks Island is allowed under a low quota and is likely not a significant pressure (Ref. 8).
Dolphin and Union Caribou
The Dolphin and Union caribou herd is unique. These caribou are morphologically similar to both barren-ground caribou and Peary caribou, representing an inter-grade between them. The majority of the herd moves on sea ice between their summer range on Victoria Island and their winter range on the mainland (Figure 1). The Dolphin and Union caribou are considered at risk particularly because of loss of sea ice with a warming climate and increasing marine shipping at key seasonal periods which could impede caribou movement between Victoria Island and the mainland (Ref. 9). The latest Dolphin and Union caribou surveys up to 2020 showed a rapid decline in numbers since 2015 (Figure 3).

Barren-ground Caribou and Porcupine Caribou
Migratory herds of caribou have long been known to fluctuate over wide ranges of abundance on a time scale of decades, based both on both scientific and traditional knowledge. The last period of high numbers in NWT herds was in the late 1980s and early 1990s, and since then most herds have experienced large declines in the 1990s and 2000s. Barren-ground caribou were listed as a Threatened species in the NWT in 2018 in response to these declines, with recovery planning and management actions put in place focussed on harvest, habitat protection, predator and other factors to promote population stabilization and recovery.
Eight migratory caribou herds have a part of or their annual range in the NWT (Figure 1). An additional herd, the Ahiak herd, also can range in the NWT in some years, however recent information on numbers and movements of this herd is limited. Three herds have ranges that occur only in the NWT (Tuktoyaktuk Peninsula, Cape Bathurst, and Bluenose-West). The ranges for three herds occur in both the NWT and Nunavut (Bluenose-East, Bathurst, Beverly, and Qamanirjuaq). The Porcupine caribou herd is a large population of caribou that ranges from Alaska to the Yukon and northwestern NWT (Figure 1). The herd is considered barren-ground caribou in Canada (Ref 13), but they are assessed as a separate geographically distinct group in the NWT (Ref 14). Porcupine caribou numbers have been increasing since the 2000s. The population was estimated to be approximately 218,000 caribou in 2017 (Ref 15). It is the only population of migratory caribou found on the mainland NWT showing no declines in the last decade.
All other barren-ground caribou herds in the NWT have experienced significant population declines in the 1990s and 2000s (Figure 4), although trends from 2018 2021 were positive with a number of herds showing stability or initial increase. The Bathurst herd continues to decline despite management efforts to reverse the trend. The latest estimate in 2021 (6,240 caribou) is down almost 98% since 1986, although the rate of decline from 2018 to 2021 slowed to 8% per year and the 2021 estimate was not statistically significantly different than in 2018 (8,207) and some indicators like the cow survival rate showed improvement. The Bluenose-West herd’s latest estimate is slightly lower (but not statistically significantly different) than the last estimates (2021 estimate of 18,440 caribou, down from 21,011 in 2018), however it has roughly stayed stable at low numbers between 2006 and 2018. The Cape Bathurst herd remains small but has increased slightly in numbers during the last decade (2021 estimate of 4,913 caribou). The small Tuktoyaktuk Peninsula herd may be showing recent signs of improvement (2021 estimate of 3,073 caribou, compared to 1,499 in 2018), however the change was not statistically significant. The Bluenose-East herd has stabilized, with a statistically non-significant increase to the 2021 estimate of 23,202, from 19,294 in 2018. The Beverly herd was last surveyed in 2018 (est. 103,372 caribou) and the estimate showed a slow decline of about 5% per year and a significant decline in female numbers from 136,000 during the previous survey in 2011. The Qamanirjuaq herd was last surveyed in 2017 (est. 288,244 caribou) and has been stable since 2014 after declining between 2008 and 2014.

There are many natural factors that can influence population changes in caribou, including insect harassment, forage quality, snow depth, predation, disturbance, climate and range dynamics (Ref. 13). Indigenous, local or community and scientific knowledge provide evidence of caribou populations naturally occurring fluctuations in size over periods of decades. There is evidence that climate-induced changes to the environment such as deeper snow, melting permafrost, altered timing of spring green-up and increasing shrub cover can negatively affect caribou and compound the cumulative effects of human-caused disturbances on caribou herd ranges (Ref. 14). These threats can slow the recovery of barren-ground caribou populations from current low numbers.
Threats caused by human activities on barren-ground caribou ranges have changed over the last sixty years (see focal point Human activities) and have increased most notably in the Bathurst herd range with mining and associated road development (Ref. 16).
Hunting practices have changed from dispersed harvesting to more concentrated harvesting in areas near communities or in areas with road access to caribou. Winter and all-season roads have made remote areas more accessible, and technologies such as snowmobiles, aircraft and all-terrain-vehicles (ATVs) have changed how caribou are hunted. There is currently no non-resident harvest of barren-ground caribou in the NWT, and resident harvest is limited to the Porcupine herd (two bull only tags in certain areas) and the Beverly herd (1 bull only tag in U/BC/01). Because of caribou declines, many herds also have harvest restrictions for Indigenous hunters that have been put in place through co-management processes (Ref 14). A portion of the Cape Bathurst herd’s range is closed to harvesting and there has been a Total Allowable Harvest in place for the Bluenose-West herd since 2007. All harvest of Bathurst caribou was substantially reduced in 2010 to 300 caribou/year and 80% bulls, then completely closed in the NWT in 2015 using a mobile caribou management zone in the NWT portion of its range. The Bathurst mobile no harvest zone is defined by locations of all collared Bathurst cows and bulls with a buffer around these locations. In some winters there has been mixing of Bathurst collared caribou with collared caribou from the Bluenose-East and Beverly herds, and portions of those herds that fall within the Bathurst no-harvest zone are also not available for harvest. Harvest of the Bathurst herd had been limited in Nunavut since 2016 and in 2019 was reduced to 10 caribou/year. Hunting pressure has been significantly reduced on the Bluenose-East herd since 2016 as the result of Total Allowable Harvest limits put in place in the NWT and Nunavut. In addition, in winters when both Bathurst herd and the Bluenose-East herd overlap, the mobile zone closing hunting for the former is resulting in a closure of the harvest for latter herd in the NWT.
Boreal Caribou
Boreal caribou live in the forested areas east of the Mackenzie Mountains, mostly in the Taiga Plains (Figure 1). They tend to live in small groups, prefer to stay within the forest year-round and do not migrate. Boreal caribou females space out for calving to reduce the risk of predation (Ref. 17, 18). The main threats to boreal caribou in the NWT are habitat loss and habitat fragmentation from human-caused disturbances (i.e. seismic lines and roads) and natural disturbances (i.e. forest fires). Boreal caribou using areas near roads and seismic lines are more vulnerable to predation and harvest. Disturbances like fire and timber harvest result in younger aged forests that are attractive to other prey species like moose. If there is enough young forest to increase the density of other prey, wolf density may also increase, leading to increased predation on boreal caribou.
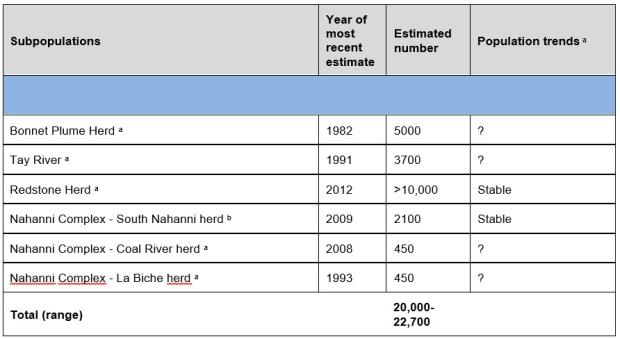
About 6,000-7,000 boreal caribou are estimated to be present in the NWT (Table 1), and are dispersed at a very low density. Population trends are estimated regularly in multiple study areas in the NWT (Ref. 19). Recent population trends in most boreal caribou study areas show stable to increasing trends, even in the southern NWT study areas where longer term trends have been decreasing and there is the most habitat disturbance. Survey information from the northernmost regions of the NWT range is not current (Ref. 19).
Traditional and community knowledge compiled in 2012 (Ref. 18) suggests that boreal caribou population trends are different depending on the area and that group sizes have been smaller in recent years than in the past.
Table1: Boreal caribou population trends in each general study area. Survey units in each study area are selected mostly for logistical reasons. These units are not equivalent to population units. Data from ECC, GNWT.

b Only two years of data are available for these study areas.
Northern Mountain Caribou
Northern mountain caribou are similar to boreal caribou but have different habitat preferences and behaviour. In the NWT northern mountain caribou live in the Mackenzie Mountains. They have distinct migrations both in elevation, where they move up or down in response to changes in food quality and availability, and in season, between summering and wintering areas (Figure 1). They sometimes gather into large groups and seasonal migrations may involve groups of thousands of animals.
There are approximately 50,000 - 55,000 northern mountain caribou in the NWT, Yukon and northern British Columbia (Ref 20). NWT population estimates total about 20,000 to 22,700 (Table 2). Northern mountain caribou numbers naturally fluctuate because of food availability, weather and predation. Human activities like hunting and habitat disturbance can add stressors that may lead to declines. In the NWT, localized threats including industrial development, roads, hunting and recreational activity are affecting caribou in some local areas, but there is uncertainty about the scale of these impacts on the NWT’s herds of northern mountain caribou. The loss of ice patches used by caribou to escape insects and cool down in the summer is also a concern (Ref 21).
NWT’s northern mountain caribou herds are not well defined and most information on population size and trend is outdated. Populations are generally thought to be stable based on scientific information, however Indigenous knowledge holders in Ross River, Yukon and in the Sahtú and Gwich’in regions have reported population declines or displacement in some areas (Ref 21).
Table 2. Northern Mountain caribou population trends and population numbers for each herd present at least partially in the NWT, based on scientific information. Data from Ref 20, Ref 21
a Low confidence in estimated numbers, trends unknown if last survey was at least 10 years ago.
b Mark/re-sight survey leading to higher confidence in numbers
Looking around
Peary caribou also occur in Nunavut. Similar weather effects, interactions between Peary caribou and muskox numbers, and movements between islands have also been noted on Nunavut’s Arctic Islands (Ref. 2).
Dolphin and Union caribou are a single population that is shared between NWT and Nunavut.
Many barren-ground caribou herds have declined in the past decades in most of northern North America. Caribou herds increased from lows in the mid-1970s to peaks in the 1990s, and declined in the 2000s. However, the timeline of population declines and peaks differs in some Alaskan and northern Quebec herds (Ref. 9).
Boreal caribou are declining throughout much of their range in North America. Threats to boreal caribou include human-caused habitat loss and fragmentation that lead to increased predation, disease, and in some areas, over-harvesting (Ref. 17).
Northern mountain caribou are in decline in the southern part of their range in Canada. Major threats are human-caused habitat change that leads to increased predation, as well as climate change (Ref. 20).
Looking forward
A slight increase in the numbers of Peary caribou observed during the 2012 survey on Prince Patrick, Melville and other northern islands signalled some recovery, and since then surveys on Banks Island and Victoria Island have showed stabilization. Warmer and snowier winters have occurred more often in the High Arctic in the past two decades than before. If such warm winters become the norm, this could impact the prospects for a full recovery of populations of Peary caribou on NWT’s northern-most islands because deep snow and ice on snow make it more difficult to Peary caribou to access food. T possibility of Peary caribou disappearing from some islands in the High Arctic remains a concern.
When barren-ground caribou herds are in decline, management actions take on much greater importance. Declining or small herds are less resilient to stressors including industrial disturbance and harvesting than herds that are increasing or larger in size. The overall goal of conservation efforts is to manage activities that humans can influence (e.g., harvesting, resource development) to support population resilience in the face of a changing climate. There have also been efforts to promote higher survival rates in calf and adult caribou by reducing wolf abundance on caribou winter ranges, with Indigenous wolf hunters playing a key role.
Management actions that have been taken to support recovery of barren-ground caribou herds have resulted from collaborative processes, many of them following regional land claims, with Indigenous government boards and co-management boards having key roles along with the territorial and federal governments. These processes include opportunities for the public and interested parties to voice their views on caribou management, including formal hearings in NWT and NU held by management boards. Given the current status of barren-ground caribou herds, there is broad public support for enhanced research, monitoring and management actions by wildlife co-management partners to support caribou conservation and recovery.
The effects of human-caused and natural habitat disturbance on boreal caribou population trends are being monitored and assessed. Boreal caribou range planning is an important part of the boreal caribou conservation efforts designed to protect critical habitat in the NWT. Overall management and recovery actions are being implemented by wildlife co-management partners, guided by collaboratively developed federal and territorial species at risk recovery strategies.
There remain a number of knowledge gaps and uncertainties about the trends of northern mountain caribou in the NWT. Further research and monitoring are needed to better understand their status and the factors affecting them. A public co-management process, with Indigenous partners, is underway to develop a cross-regional community conservation plan for northern mountain caribou (Ref. 22).
Find out more
Porcupine Caribou Management Board www.pcmb.ca
Beverly and Qamanirjuaq Caribou Management Board http://www.arctic-caribou.com/
Advisory Committee for Cooperation on Wildlife Management https://accwm.com/
CARMA (CircumArctic Rangifer Monitoring and Assessment Network) https://carma.caff.is/
NWT Species at Risk www.nwtspeciesatrisk.ca
GNWT ECC - Caribou in the NWT https://www.ecc.gov.nt.ca/en/services/caribou-nwt
- See SPECIES AT RISK for indicators on the status of each type of caribou under species at risk legislation.
- See CLIMATE for indicators on weather events that affect Peary caribou and muskox in the High Arctic.
Technical Notes
Dolphin and Union caribou:
The original aerial survey estimates for Dolphin and Union caribou from 1994 onwards were modified with a correction factor, to account for caribou that were outside the survey zone to produce the extrapolated population estimates that are shown in the graph (Refs 10, 11).
Barren-ground caribou:
Population data prior to 1980s were collected using visual surveys. Photographic surveys were used from the 1980s onward. For eastern NWT barren-ground herds such as the Bluenose-East and Bathurst, as well as the Qamanirjuaq herd in NU, population estimates are derived from photographic surveys in June when female caribou are concentrated on the calving grounds. An overall herd estimated is extrapolated from the estimated numbers of females from the calving ground surveys and sex ratios from fall composition surveys to account for males that are rarely on the calving grounds. Caribou in the western NWT herds like the Tuktoyaktuk Peninsula, Cape Bathurst, Bluenose-West and Porcupine, are counted from aerial photographs taken over large post-calving aggregations that form for insect relief. When caribou herds are declining, the GNWT conducts population surveys more frequently and other monitoring is also increased.
Up until the 1994 estimate, the Beverly herd was defined as calving inland on a calving ground south of Garry Lakes in Nunavut. This calving ground has not been used since 2009. The fate of that Beverly herd is unclear; it either shifted in large numbers to calving in the Queen Maud Gulf lowlands (Nagy et al. 2011) or declined to very low numbers and the remnant herd joined a larger herd calving in the Queen Maud Gulf (Adamczewski et al. 2015). The Beverly herd defined more recently as calving in the central and eastern Queen Maud Gulf (2011 and onward) may not correspond exactly to the earlier inland-calving Beverly herd. Given uncertainties as to the fate of the herd that calved on the Beverly South calving ground up to about 2009, estimates of the Beverly herd from 2011 onward are not directly comparable to the earlier estimates. This uncertainty is shown in the graph with two different colours.
The population trajectory of the Qamanirjuaq herd from 1994 to 2008 is uncertain because of the long period of time between surveys.
Boreal caribou:
It is difficult to obtain population estimates of boreal caribou based on their low population density and detectability. Trends are based on annual survival rates of collared adult females and spring composition surveys determining calf recruitment rates. Each survey year, a finite rate of population change (λ; lambda) is determined using a stochastic version of equation [λ=adult female survival/(1-female calf recruitment)] (Ref. 19). Due to substantial annual variations in adult female survival and calf recruitment, lambda values are averaged over many years to provide an indication of whether the caribou population trend in each study area is increasing, stable or declining. In this indicator, if average lambda >1.01, the population is noted as “increasing”, average lambda = 0.99-1.01, it is reported as “stable”, and average lambda <0.99, is indicated “declining”.
References
Ref. 1. Species at Risk Committee. 2012. Species Status Report for Peary Caribou (Rangifer tarandus pearyi) in the Northwest Territories. Species at Risk Committee, Yellowknife, NT. Available at www.nwtspeciesatrisk.ca
Ref. 2. COSEWIC. 2015. COSEWIC assessment and status report on the Peary Caribou Rangifer tarandus pearyi in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xii + 92 pp.(http://www.registrelep-sararegistry.gc.ca/default_e.cfm).
Ref. 3. Davison, T., Williams, J. 2012. Caribou and muskoxen survey on Melville and Prince Patrick Islands, 2012 summary. Department of Environment and Climate Change, GNWT Inuvik Region, Inuvik, NWT.
Ref. 4. Miller F. L., Gunn A. 2003. Status, population fluctuations and ecological relationships of Peary caribou on the Queen Elizabeth Islands: Implications for their survival. Rangifer 14:213-226
Ref. 5. Nagy J. A., Larter N. C., Fraser V. P. 1996. Population demography of Peary caribou and muskox on Banks Island, N.W.T., 1982-1992. Rangifer 16(4):213-222
Ref. 6. Environment Canada. 2004. Resolute CARS Historical Weather. Available at http://www.climate.weatheroffice.ec.gc.ca/advanceSearch/searchHistoricDa... . EC, Canada.
Ref. 7. Grenfell T. C., Putkonen J. 2008. A method for the detection of the severe rain-on-snow event on Banks Island, October 2003, using passive microwave remote sensing. Water Resources Research 44:W03425
Ref. 8. Government of the Northwest Territories. 2018. Summary of Harvest Data for Species in the Inuvialuit Settlement Region: July 2013 to June 2018. Government of the Northwest Territories, Environment and Climate Change. Inuvik Region.
Ref. 9. Species at Risk Committee. 2013. Species Status Report for Dolphin and Union Caribou (Rangifer tarandus groenlandicus x pearyi) in the Northwest Territories. Species at Risk Committee, Yellowknife, NT. Available at www.nwtspeciesatrisk.ca
Ref. 10. Management Plan for the Dolphin and Union Caribou (Rangifer tarandus groenlandicus x pearyi) in the Northwest Territories and Nunavut. 2018. https://www.nwtspeciesatrisk.ca/sites/enr-species-at-risk/files/dolphin_...
Ref. 11 Leclerc, L-M., Boulanger, J. 2020. Population Estimate of the Dolphin and Union Caribou herd (Rangifer tarandus groenlandicus x pearyi) Coastal Survey, October 2018 and Demographic Indicators. Nunavut Department of Environment, Wildlife Research Section, Kugluktuk, NU. https://www.gov.nu.ca/sites/default/files/final_report_2018_dolphin_and_...
Ref. 12. Campbell. M. Ringrose, J., Boulanger, J. Roberto-Charron, A., Methuen, K. Mutch, C. Davison, T. and Wray.C. 2021. An aerial abundance estimate of the Dolphin and Union Caribou (Rangifer tarandus groenlandicus x pearyi) herd, Kitikmeot Region, Nunavut–Fall 2020, GN Department of Environment, 44 pp.
Ref. 13. COSEWIC. 2016. COSEWIC assessment and status report on the Caribou Rangifer tarandus, Barren-ground population in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xiii + 123 pp. (http://www.registrelep-sararegistry.gc.ca/default.asp?lang=en&n=24F7211B-1).
Ref. 14. Species at Risk Committee. 2017. Species Status Report for Porcupine Caribou and Barren-ground Caribou (Tuktoyaktuk Peninsula, Cape Bathurst, Bluenose-West, Bluenose-East, Bathurst, Beverly, Ahiak, and Qamanirjuaq herds) (Rangifer tarandus groenlandicus) in the Northwest Territories. Species at Risk Committee, Yellowknife, NT. Available at www.nwtspeciesatrisk.ca
Ref. 15. Porcupine Caribou Technical Committee. 2021. Porcupine Caribou Annual Summary Report 2019-2020. Submitted to Porcupine Caribou Management Board. Available at www.pcmb.ca
Ref. 16. GNWT. 2019. Bathurst Caribou Range Plan. 2019. Environment and Climate Change, Government of the Northwest Territories, Yellowknife, NT. 86 + iii pp. https://www.ecc.gov.nt.ca/sites/ecc/files/resources/bathurst_caribou_range_plan_2019_-_plan_pour_laire_de_repartition_des_caribous_de_bathurst_2019.pdf
Ref. 17. COSEWIC. 2014. COSEWIC assessment and status report on the Caribou Rangifer tarandus, Newfoundland population, Atlantic-Gaspésie population and Boreal population, in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xxiii + 128 pp. (www.registrelep-sararegistry.gc.ca/default_e.cfm).
Ref. 18. SARC. 2012. Species Status Report for Boreal Caribou (Rangifer tarandus caribou) in the Northwest Territories. Species at Risk Committee, Yellowknife, NT. 145 pp. Available at www.nwtspeciesatrisk.ca
Ref. 19. GNWT. 2019. A Framework for Boreal Caribou Range Planning. Environment and Climate Change, Government of the Northwest Territories, Yellowknife, NT. 87 pp. https://www.ecc.gov.nt.ca/sites/ecc/files/resources/boreal_caribou_range_planning_framework_2019_-_cadre_de_planification_de_laire_de_repartition_du_caribou_boreal_2019.pdf
Ref. 20. COSEWIC. 2014. Update COSEWIC report on the Caribou (Rangifer tarandus) in the Northern Mountain Central Mountain and Southern Mountain designatable units. Environment Canada. 109 pp.
Ref. 21. SARC. 2020. Species Status Report for Northern Mountain Caribou (Woodland Caribou [Northern Mountain Population]) (Rangifer tarandus caribou) in the Northwest Territories. Species at Risk Committee, Yellowknife, NT. Available at www.nwtspeciesatrisk.ca
Ref. 22. Nıo Ń ę P’ęné Working Group. 2019. Nío Nę P’ęnę́Begháré Shúhta Goɂepé Narehɂá – Trails of the Mountain Caribou Plan [DRAFT]. Proposed joint mountain caribou plan. Available at https://www.srrb.nt.ca/index.php?option=com_docman&view=download&alias=1833-19-06-n-o-ne-p-ene-trails-of-the-mountain-caribou-plan-draft-pdf&category_slug=3-3-relevant-documents-shu-hta-go-epe-mountain-caribou&Itemid=697
Red. 23.NWT CIMP (NWT Cumulative Impact Monitoring Program. 2021.Asessing trends in caribou observations and health. NWT Environmental Research Bulleting.7 (32). Available at https://www.ecc.gov.nt.ca/sites/ecc/files/resources/128-cimp_bulletin_32_en.pdf
15.2 Trends in birds
This indicator tracks population changes of NWT bird species. The information for this indicator is obtained, with permission, from the report “The State of Canada’s Birds 2019” (Ref. 1).

This indicator was prepared by the Government of the Northwest Territories, Department of Environment and Climate Change, using information obtained from the report “The State of Canada’s Birds 2019” (Ref. 1, with permission).
State of birds reports are produced by the North American Bird Conservation Initiative (NABCI-Canada), under the leadership of Environment and Climate Change Canada, Bird Studies Canada, Ducks Unlimited Canada, and Nature Canada. The data are mostly from the Christmas Bird Counts (CBCs), Breeding Bird Surveys (BBS) and other surveys such as US Fish and Wildlife Service’s waterfowl aerial surveys and shorebird surveys in the Arctic (PRISM program). See the technical note below for more information on bird monitoring programs.
NWT Focus
The two major biomes of the NWT, the tundra and the taiga-boreal forest, are home to 300 species of birds. The vast majority are migratory species that spend more than eight months each year outside the NWT during migration and on their wintering grounds. Only about 6% of bird species are resident species that remain in the NWT year-round. Birds have important roles in northern ecosystems.
Ducks, geese, grouse, and ptarmigans are essential food sources for many northern families. Waterfowl hunting is part of peoples’ traditional link to the land and many families travel seasonally to good bird hunting areas every year. Hunters track the numbers and health of these harvested bird species closely.
Songbirds, shorebirds, and woodpeckers are major predators of insects, including insect pest species. They contribute to plant seed dispersal, are prey to other species, and in the case of woodpeckers, provide homes for other species.
Falcons, eagles, owls, and other raptors are top predators. Monitoring populations of top predators offers insights into the health of ecosystems, as they are susceptible to pollutants and to changes in prey populations. Monitoring the status of fish-eating birds (e.g. loons, pelicans) and marine birds (e.g. sea ducks) helps us to understand changes in aquatic and marine ecosystems.
Current View: status and trend
In Canada, aerial insectivores, grassland birds, and shorebirds are in steep decline (Ref. 1). Most of these species have declined by more than half compared to the numbers seen in the 1970s.
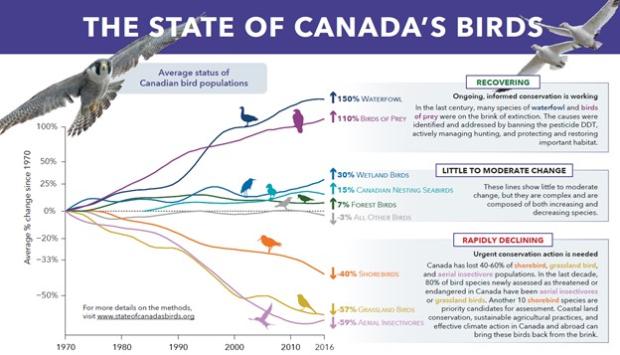
The reasons for these declines are unclear, but potential threats across the Americas are being investigated and include pollution, habitat loss, over-harvesting, insect controls leading to declines in insect populations, and climate change. Little is known about specific drivers of population change in the NWT.
Many migratory species in Canada are declining and there is growing concern that this is indicative of widespread changes in our ecosystems. For example, there is evidence of declines of most shorebirds that nest in the Arctic, some shorebirds in the taiga and boreal forest (Ref. 2), and many forest bird species that specialize in aerial feeding on insects (Ref. 3, 5). Further studies are needed to determine the main reasons for these declines in population number, but climate change (Ref. 6), habitat loss across breeding, non-breeding, and migration ranges, and declines in insect populations may be involved.
Some species have increased in numbers over the past few decades. For example, the number of lesser snow geese nesting in the western Canadian Arctic (mostly on Banks Island) (Ref. 7) has more than doubled since the 1970s mostly due to increased availability of food at migratory stopover sites and wintering grounds as a result of changes in agricultural practices (Ref. 7).
Looking around
A collaborative approach to research and monitoring is essential to understanding why some migratory birds are declining across North America, to reduce threats, and to help populations recover. Management and conservation strategies, such as the North American Bird Conservation Initiative, are being implemented by agencies responsible for the management of birds in the NWT, Canada, as well as in the US and Mexico.
Find out more
Go to The State of Canada’s Birds 2019 at http://nabci.net/wp-content/uploads/39-004-Canada-State-of-Birds_EN_WEB-2.pdf
Technical Notes
The Breeding Bird Survey (BBS) tracks species on their breeding grounds. There are five survey sites in the NWT. The Christmas Bird Count (CBC), a monitoring program that has been conducted for over 100 years, collects data on birds on bird wintering ranges. There are usually about five to six sites in the NWT. Trends in ptarmigans, ravens and other species that winter in the NWT can be tracked using this volunteer program. As most NWT birds migrate south for winter, the population trends of these species are determined from CBC sites in southern Canada and the United States. The BBS and CBC programs are administered in Canada by Bird Studies Canada and provide the majority of data to determine population trends on most small birds nesting in the NWT.
To learn more go to https://www.birdscanada.org/
The NWT/Nunavut Bird Checklist is part of the eBird initiative to track sightings of birds around the world, including in NWT’s ecosystems. Some NWT birds migrating further south to the US, Mexico and central-south America, are monitored by the Canadian Migration Monitoring Network at a few stations in Canada, the US, and elsewhere. To learn more go to https://ebird.org/home.
Continental populations of ducks and geese are monitored by the US Fish and Wildlife Service in collaboration with the Canadian Wildlife Service. Data are used to track the status of waterfowl and adjust hunting regulations. Waterfowl surveys are part of the North American Waterfowl Management Plan.
Some monitoring programs have been designed to collect data on some species of birds requiring special attention in the NWT and elsewhere. The state of NWT shorebirds is tracked by the Program for Regional and International Shorebird Monitoring (PRISM) as part the Shorebird Conservation Strategy and Action Plan. Monitoring of raptor nesting sites in the NWT is done using the NWT-NU Raptor Database. Peregrine Falcons are monitored every five years in two study areas in the NWT as part of the North American Peregrine Falcon Surveys.
References
Ref. 1. North American Bird Conservation Initiative Canada. 2019. The State of Canada’s Birds, 2019. Environment and Climate Change Canada, Ottawa, Canada. 12 pages.
Ref. 2. Hope, D.D, C. Pekarik, M.C. Drever, P.A. Smith, C. Gratto-Trevor, J. Paquet, Y. Aubry, G. Donaldson, C. Friis, K. Gurney, J. Rausch, A.E. McKellar & B. Andres. 2019. Shorebirds of conservation concern in Canada – 2019. Wader Study 126(2): 88–100.
Ref. 3. Nebel, S., A. Mills, J. D. McCracken, and P. D. Taylor. 2010. Declines of aerial insectivores in North America follow a geographic gradient. Avian Conservation and Ecology 5(2):1
Ref. 5. Blancher P. 2003. The Importance of Canada's Boreal Forest to Landbirds. Canadian Boreal Initiative and the Boreal Songbird Initiative.
Ref. 6. Audubon. 2020. Survival by Degree: 389 Bird species on the brink. Available at https://www.audubon.org/climate/survivalbydegrees.
Ref. 7. Canadian Wildlife Service Waterfowl Committee. 2019. Population Status of Migratory Game Birds in Canada (and Regulation Proposals for Overabundant Species). November 2019. Canadian Wildlife Service, Environment Canada, Ottawa, ON.
15.3. Trends in range expansions of mammals
This indicator tracks mammal species that are newly introduced or expanding their current ranges into the NWT.

This indicator was prepared by the Government of the Northwest Territories, Department of Environment and Climate Change, using information collected from NWT residents and visitors, and tracked using the NWT General Status Ranking Program (Ref. 1). Official species lists have been compiled for the NWT General Status Ranking Program since 2000 and include all mammal species in the NWT.
Every year, sightings reported by the public are used to update species range maps. Range expansions of mammals in the NWT are tracked using the ecosystem-based automated range mapping method (Ref. 2), based on ecoregion level IV, as described in the NWT’s Ecosystem Classification (Ref. 3).
The three categories are:
Vagrant = species occurring infrequently and unpredictably in the NWT. These species are outside their usual range and may be in the NWT due to unusual weather occurrences, an accident during migration, or unusual behaviour by a small number of individuals.
New – range extension into NWT = species newly discovered in the NWT, for which there is evidence of recent range extension. This definition helps track species that have recently established themselves in the NWT (“true” new species), as their pattern of distribution changes.
Range extension within NWT = species already present in the NWT, for which there is evidence of recent extension into habitats or ranges not known to be previously occupied since the last glaciation about 7000 to 10,000 years ago. This definition helps track changes in mammal distributions in the NWT due to, for example, population increases, climate change, and habitat changes.
NWT Focus
Changes in species composition and range expansion are a predicted outcome of a changing climate. Tracking these changes using the NWT’s ecoregions provides a method to track in a systematic manner.
Current View: status and trend
Ten species of mammals have experienced changes in their ranges in the past 15 years. Most movements are from south to north, but some species, such as muskox on the mainland, have expanded their range from northern tundra habitats to southern forested areas (Table 1).
Detecting true range expansions can be difficult for less studied species. For example, three species of bats (long-legged myotis, eastern red bat, and big brown bat) were first documented in the NWT in 2006, but because there had been very few studies on NWT bats previously, it is not known whether these were new range extensions, or the species had been present before but not noted (Ref. 11).
Table 1. Species with known range expansions, category of range expansion, and comments including details on evidence, movements between ecozones. Overall source: Information from the NWT General Status Ranking Program and as referenced.


Looking forward
Tracking range expansion in Canada is now easier on the iNaturalist.ca platform, using the project “Canada’s Groundbreaking Observations” (Ref. 12).
Automated wildlife cameras and other recording devices designed to track sounds are being deployed in almost every ecozone of the NWT. These devices compliment conventional wildlife surveys and offer economical and low disturbance options for recording mammal presence in an area. The use of automated cameras by wildlife management agencies, wildlife co-management partners, communities and the general public provides opportunities to help with tracking presence, movements and range expansions of mammal species in the NWT.
As people report species of mammals that have previously been rarely or not seen before, our knowledge of NWT mammals and their distribution will increase.
Overall, some species are being seen farther north than before. This movement northward is not occurring at a constant rate. Some years more observations of range expansions are reported than in other years. Vagrancy, where animals are seen temporarily a long distance from where they normally occur, is normal in mammals. Individuals, especially young ones, will try new territories and explore new habitats, and can travel farther than expected. It remains difficult to detect when there is an increase in vagrancy and when populations of a new species are established in a new area, comprising a range extension.
All NWT residents have a role to play in monitoring range extension of mammals by recording and reporting details on observations of species that are in a “new place” to ECC at wildlifeobs@gov.nt.ca.
Find out more
For more information on the NWT General Status Ranking Program go to https://www.ecc.gov.nt.ca/en/services/biodiversity/nwt-species-general-s....
For more information on NWT’s ecozones go to https://www.ecc.gov.nt.ca/en/services/ecosystem-classification.
References
Ref. 1. Working Group on General Status of NWT Species. 2021. NWT Species General Status Ranking, Environment and Climate Change, GNWT. Yellowknife, GNWT. Available at: https://www.ecc.gov.nt.ca/en/services/biodiversity/nwt-species-general-s...
Ref. 2. NatureServe Canada. 2021. EBAR Mapping. Available at: https://www.natureserve.org/natureserve-network/canada/biodiversity-data...
Ref. 3. Environment and Climate Change. 2013. Ecosystem Classification. Available at:https://www.ecc.gov.nt.ca/en/services/ecosystem-classification.
Ref. 4. NWT Species at Risk Program. 2021. Wood Bison. Available at: https://www.nwtspeciesatrisk.ca/species/wood-bison
Ref. 5. Environment and Climate Change. 2021. Muskoxen. Available at: https://www.ecc.gov.nt.ca/en/services/muskoxen
Ref. 6. Veitch A.M. 2001. An Unusual Record of a White-tailed Deer, Odocoileus virginianus, in the Northwest Territories. Canadian Field-Naturalist 115:172-175.
Ref. 7. Canadian Broadcasting Corporation. 2018. Coyote believed first sighted in Beaufort Delta region since 1974. Available at: https://www.cbc.ca/news/canada/north/rare-coyote-sighting-beaufort-delta...
Ref. 8. Gau, R., R. Mulders, T. Lamb and L. Gunn. 2001. Cougar (Puma concolor) in the Northwest Territories and Wood Buffalo National Park. Arctic 54, 185-187.
Ref. 9. Communities Inuvialuit Region. 2005. Unikkaaqatigiit – Putting the Human Face on Climate Change: Perspectives from the Inuvialuit Settlement Region. Joint publication of Inuit Tapiriit Kanatami, Nasivvik Centre for Inuit Health and Changing Environments at Université Laval and the Ajunnginiq Centre at the National Aboriginal Health Organization. Ottawa, ON.
Ref. 10. Doupe J.P., J.H. England, M. Furze and D. Paetkau. 2007. Most Northerly Observation of a Grizzly Bear (Ursus arctos) in Canada: Photographic and DNA Evidence from Melville Island, Northwest Territories. Arctic 60:271-276.
Ref. 11. Lausen, C. 2006. Bat Survey of Nahanni National Park Reserve and Surrounding Areas, NWT. Prepared for Parks Canada and Canadian Parks and Wilderness Society, NWT Chapter. 39pp. Available at: http://cpawsnwt.org/uploads/BAt_SurveyReport_Lausen_26NovemberFINAL.pdf
Ref. 12. Web. 2021 Available at: https://inaturalist.ca/projects/canada-s-groundbreaking-observations
Ref. 13. Pongracz, J. D., Paetkau, D., Branigan, M., & Richardson, E. 2017. Recent hybridization between a polar bear and grizzly bears in the Canadian Arctic. Arctic, 151-160.
Ref. 14. Winbourne, J., and Benson, K. 2021. Species status report (Traditional and community knowledge component) for muskoxen (Ovibos moschatus) in the NWT. 92 pp. Available at https://www.ecc.gov.nt.ca/sites/ecc/files/resources/species_status_report_-_traditional_and_community_knowledge_component_for_muskoxen_in_the_nwt_292_manuscript.pdf
15.4 Trends in alien species in the NWT
This indicator tracks the species (in selected species groups) that have been introduced in the NWT.

This indicator tracks introduced or alien species that are now found living in the wild in NWT ecosystems, are not captive, and can survive in our northern ecosystems without continuous care by humans.
Past introduction of species into the wild that did not survive are not part of the indicator.
This indicator helps track alien and introduced species using the following definitions.
Alien Species = Species with natural ranges outside North America that have been intentionally or unintentionally introduced due to human activities and have been found to survive in the wild in the NWT. All alien species are not originally native to North America and NWT.
Introduced Species = Species with natural ranges outside the NWT that have been intentionally or unintentionally introduced due to human activities and have been found to survive in the wild in the NWT.
This indicator was prepared by the Government of the Northwest Territories, Department of Environment and Climate Change, using information from the NWT Species General Status Ranking Program (Ref. 1). Species groups are selected for this indicator if they have been tracked for three or more reporting cycles, i.e., for 15 years or more. Official species lists have been compiled for the NWT General Status Ranking Program (Ref. 1) since 2000. Updates on introduced alien species in the NWT are only possible with the contribution of observations of many NWT residents and tourists interested in NWT biodiversity.
This indicator is an updated version of the archived indicator Number of introduced mammal, bird and fish species in the NWT.
NWT Focus
Changes in the number or percentage (compared to native species) of alien species are monitored as their presence may affect the NWT’s ecosystems. Increases in the number of alien species can signal changes in human activities, but also can be indicating that our northern weather or environment no longer prevents some species from surviving in the wild and these species are becoming more integrated into NWT ecosystems.
Current View: status and trend
Trends
The percentage of alien species has not increased for most groups in the past 20 years (Figure 1). The percent of vascular plants species that are alien to the NWT is increasing by approximately 1 % every 5 years (Figure 1). This increase may be due to enhanced monitoring, actual increases through intentional or unintentional introductions of vascular plants in the NWT, or both. Many of these new alien vascular plants are found during invasive plant roadside surveys (Ref. 10).

Notes on alien wildlife species
Birds
Three bird species (Rock Pigeon, House Sparrow, and European Starling) were introduced to North America from Eurasia and are present today in the NWT. They may survive on their own or may be assisted by staying near heat refugia in communities to survive during winters.
Rock Pigeon (Columba livia) was introduced to North America in the early 1600s (Ref. 3). It is not known when they were first introduced into the NWT. The species is present in the wild in the NWT only around Fort Smith (southern part of the Taiga Plains) and appears to survive only if fed and provided some shelter.
The House Sparrow (Passer domesticus) is present in and near many of the larger NWT communities. They do not seem to be able to survive in natural habitats that have not been modified by humans. Their population numbers in Yellowknife have large fluctuations (Ref. 4). This species was introduced to North America from England in late 1800s (Ref. 5) and quickly expanded its range. It is not known when they were first introduced into the NWT.
The European Starling (Sturnus vulgaris) is present in communities south of Great Slave Lake and is also seen in Yellowknife. This bird is not able to survive far from habitats that have been modified by humans. The species was introduced to North America in 1890 (Ref. 6) and quickly expanded its range. It is not known when they were first introduced into the NWT. Like the House Sparrow, European Starlings are known to destroy nests of other cavity-nesting species, but the impacts of this on NWT native birds are not well understood.
Fish
Intentional fish introductions, or fish stocking, has been used in the NWT in the past to enhance the fishing potential of a small number of lakes, providing more opportunities for sport fishing near roads. All known fish introductions were carried out as part of stocking initiatives (Ref. 7). Fish stocking has not occurred in the NWT since 1990.
Rainbow Trout (Oncorhynchus mykiss) were introduced from other watersheds in North America to Seven Mile Lake (taiga plains) in 1959 (Ref. 7). This species was unsuccessfully stocked into Lake Nine, near Polar Lake (year uncertain) and in a borrow pit south of Hay River (taiga plains). Many introductions occurred in Polar Lake, near Hay River, starting in 1977 (Ref. 7) and more recently in the summer 2011 10000 rainbow trout (triploids) were stocked for use by an angling group in Hay River. Rainbow trout were also introduced into Upper Cabin Lake, on the Ingraham Trail (taiga shield) in 1982, 1985, and 1990. There was no sign of reproduction. It is not known if introduced rainbow trout populations are still present, but it may be possible.
There were previous attempts to introduce Brook Trout (Salvelinus fontinalis) from Alberta into Seven Mile Lake (1949), Little Buffalo River (1960-61) and Polar Lake (1971). The viability of this species in the NWT is unknown (Ref. 1).
One species, Arctic Char (Salvefinus alpinus), is native to the NWT, but some fish were introduced outside of its normal range. Arctic char were introduced in 1989 from Tree River, Nunavut into several lakes near Yellowknife (taiga shield) and Hay River (taiga plains), including Polar Lake, where the species persists today (Ref. 7).
Attempts to stock other species of freshwater fish in lakes in the NWT have not been successful. Cutthroat Trout (Oncorhynchus clarki) and Splake (Salvelinus namaycush x Salvelinus fontinalis), a hybrid between lake Trout and Brook Trout, were introduced into Seven Mile Lake (1964), but the introduction was unsuccessful (Ref. 7). Brook trout (or splake) was introduced into Polar Lake (1971) but are not present there today. These introductions are noted for completeness, but are excluded from the indicator as they did not result in viable populations.
Mammals
In 2020, the only alien mammal known to survive with little or no human help is the Domestic Rabbit (Oryctolagus cuniculus) in Fort Smith, Taiga Plains.
Horses have been introduced to the NWT, but they are not included in the list of introduced species as all are pack animals requiring some care to survive. Some horses have been known to escape in the past but did not survive to reproduce. Six geldings were observed within the range of the Mackenzie bison population north and west of Fort Providence in the late 1980s and early 1990s. Only one was still alive in 2007, and none have been seen since then.
Some wild boars (Sus scrofa) were released in 1996 near Fort Resolution in the southern Taiga Plains. All released animals were removed a few weeks later. No further observations of wild boar have been detected near Fort Resolution since then. In 2010, one wild boar was seen near Enterprise. The source of this animal is unknown and it is not known if it was part of a larger group. However, no other wild boars have been observed in the NWT since that time.
In 1935, after a five-year trek from Nome, Alaska, 2370 reindeer were herded to the NWT and arrived in the Mackenzie Delta (Ref. 8). A privately-owned, free-ranging herd of reindeer can still be found on the Tuktoyaktuk Peninsula today. Reindeers are the same species as caribou (Rangifer tarandus), so they are not included in the list of introduced mammal species, but these particular animals are of a subspecies not native to the NWT.
Other species
The percentage of alien species in some species groups in the NWT is high (Table 1). For example, most of the earthworms and the only species of harvestman present in the NWT have been introduced with soil or other gardening and agricultural products (Ref.1).
Table 1. Number of alien species and total number of species known to be present in NWT, per species group with at least one alien species. Source: NWT Species General Status Ranking Program, Ref.1.


The Grey Fieldslug (Deroceras reticulatum), a species of terrestrial snail originating from Europe, was accidentally introduced to gardens in North America. The first record of an introduced mollusc in the NWT was in Hay River in 2013, they were also found in Yellowknife in 2017 (Ref. 9).
The Cabbage White (Pieris rapae) butterfly, the bane of any gardener who intends to grow broccoli, cabbage, or kale, has been reported in fair numbers in Yellowknife, Hay River and Fort Simpson, and is likely to be present in most NWT communities with gardens or green-houses (Ref. 1). This alien butterfly was first introduced to North America accidentally around 1860 from Eurasia.
Looking forward
Tracking alien species in a systematic manner requires official taxonomic lists. For many groups, taxonomic lists for the NWT were started in 2021 (e.g., annelids, flies, true bugs, springtails, micro-moths, sawflies). Monitoring programs for soil arthropods, micro-insects, and garden or agricultural pests are also required, but have yet to be completed in the NWT.
Some wildlife species thrive near humans. The number of introduced species in the NWT is expected to increase if more habitats become fragmented, because many species introduced by humans survive better near communities or in habitats created by humans.
The number of introduced species in the NWT is very small compared to other jurisdictions further south. NWT’s climate makes survival a challenge unless species are adapted to the North or humans provide some help in the form of shelter and food. With a warming climate more invasive species may become established in both disturbed and undisturbed habitats in the NWT. All residents and visitors have a role to play in preventing unintentional releases of alien species that may become naturalized and negatively affect the NWT’s ecosystems.
Find out more
For more information on the NWT General Status Ranking Program go to www.nwtspeciesatrisk.ca
See Climate for other indicators on climate in the NWT. See Vegetation for indicators related to alien vascular plants and pest species affecting our forests. See Human Activities and Landscape Changes for indicators on short-term and long-term changes to ecosystems that may result in an increased number of alien or introduced species.
References
Ref. 1. Working Group on General Status of NWT Species. 2021. NWT Species General Status Ranking program Yellowknife, GNWT. Available at https://www.ecc.gov.nt.ca/en/services/biodiversity/nwt-species-general-s...
Ref. 2. ECC. 2020. Invasive alien species program. Available at https://www.ecc.gov.nt.ca/en/services/invasive-alien-species
Ref. 3. Johnston R.F. 1992 Rock Pigeon (Columba livia). in The Birds of North America Online, Ed. (A.Poole, Ed. Cornell Lab of Ornithology, Ithaca.
Ref. 4. Audubon Society. 2021.Christmas Bird Count. Available at https://www.audubon.org/conservation/science/christmas-bird-count
Ref. 5. Lowther P.E., C.L. Cink. 2006 House Sparrow (Passer domesticus) in The Birds of North America Online (A. Poole, Ed.) Cornell Lab of Ornithology. http://bna.birds.cornell.edu/bna/species/012
Ref. 6. Cabe P.R. 1993 European Starling (Sturnus vulgaris). in The Birds of North America Online, Ed. A. Poole, Ed. Cornell Lab of Ornithology; Ithaca.
Ref. 7. Crossman E.J.1991. Introduced Freshwater Fishes: a Review of the North American Perspective with Emphasis on Canada. Canadian Journal of Fishery Aquatic Science 48 (Suppl. 1):46-57.
Ref. 8. Treude. E. 1979. Forty years of reindeer herding in the Mackenzie delta, N.W.T. Polar Geo. 3(3): 121-138.
Ref. 9. INaturalist. 2021 Available at https://inaturalist.ca/
Ref.10. Oldham M.J., and Delisle-Oldham, M. 2017. Report on the 2016 survey of exotic plants along Northwest Territories highways. GNWT, Yellowknife. 53 pp.
15.5. Status of wildlife health in terrestrial environments
This indicator tracks important pathogens of NWT wildlife that may impact wildlife or human health, and provides an overview of wildlife health research and monitoring conducted in the NWT.
Wildlife health is broadly defined as the resilience of individuals and their capacity to cope with stressors, leading to long-term sustainability of wildlife populations (Ref. 1). This definition recognizes that health is not merely the ‘absence of disease’ but depends on multiple interacting biological, social, and environmental factors.

Wildlife health can be monitored at both individual animal and population scales. The health of individual animals includes the presence or absence of certain pathogens (see glossary of terms in the Technical Notes), disease, contaminants (see indicators in Contaminants focal point), stress, immune function, physiological and nutritional status, and changes to body condition (see health metrics table in Technical Notes).
Health at the population level can be assessed using changes in population or group size, demographic information (e.g., pregnancy rates, calf-cow ratios, mortality and survival rates), distribution, and population trends. Population trends for caribou are tracked in the Wildlife focal point.
The focus of this indicator on the status of wildlife health is on individual animal health, which can of course have broader impacts on the health of populations.
Approaches that have been applied in the NWT to monitor wildlife health include enhanced active and passive targeted health surveillance programs, targeted wildlife health and disease research, community-based monitoring, and documentation of local and Traditional Knowledge (Ref. 2). Drawing on different knowledge systems and information sources helps broaden our understanding of wildlife health status and historical trends, and community-based monitoring programs can augment spatial and temporal coverage given the large size of the NWT (Ref 3, 4).
The information presented in this indicator is a compilation of work done by the Department of Environment and Climate Change in collaboration with a wide range of partners from other agencies, Indigenous governments, Indigenous organizations, renewable resources boards, non-governmental organizations, communities and individuals conducting wildlife health research and monitoring in the NWT.
This indicator was prepared by a group of wildlife health specialists from academia, including the University of Calgary and the Government of the Northwest Territories, Department of Environment and Climate Change. The information presented in this indicator is a compilation of work conducted in collaboration with a wide range of partners from other agencies, Indigenous governments, Indigenous organizations, renewable resources boards, non-governmental organizations, communities and individuals conducting wildlife health research and monitoring in the NWT.
This indicator expands on the archived “Trends in winter tick in moose” published in 2015.
NWT Focus
Wildlife are an important social, cultural, and economic resource for communities in the NWT (Ref. 5). Given the importance of wildlife as a nutritional resource, access to healthy wildlife is important to support food security. Wildlife is important for physical health and cultural resilience for all NWT residents, especially for Indigenous peoples, and as a result, wildlife health and public health are intimately intertwined.
Many factors affect wildlife populations, including a range of pathogens, parasites, contaminants and stress. Changes in wildlife health can also be an early warning sign of ecosystem and environmental change. Monitoring wildlife health is important to identify emerging wildlife health issues that might impact wildlife conservation and public health, to prevent the introduction or spread of new or emerging wildlife diseases, and to respond to individual cases and outbreaks in a timely way (Ref. 4).
Both anthropogenic (human-caused) and natural ecosystem changes can affect wildlife health in the NWT. Climate change, as a major driver of northern ecosystem change, is altering primary production and food webs, and can facilitate the emergence and expansion of some parasites and diseases (Ref. 6, 7). These changes could have potential implications on the health, reproduction and survival of wildlife, or indirect effects on fitness via reduced body condition or increased susceptibility to predation (Ref. 8). Zoonotic diseases and parasites (those pathogens that can be transmitted to people) and contaminants in wildlife can have implications for food safety and security of northern residents who rely on country foods. Wildlife can also act as reservoirs for pathogens that can be transmitted to domestic animals, including pets, and livestock.
Current view: status and trend
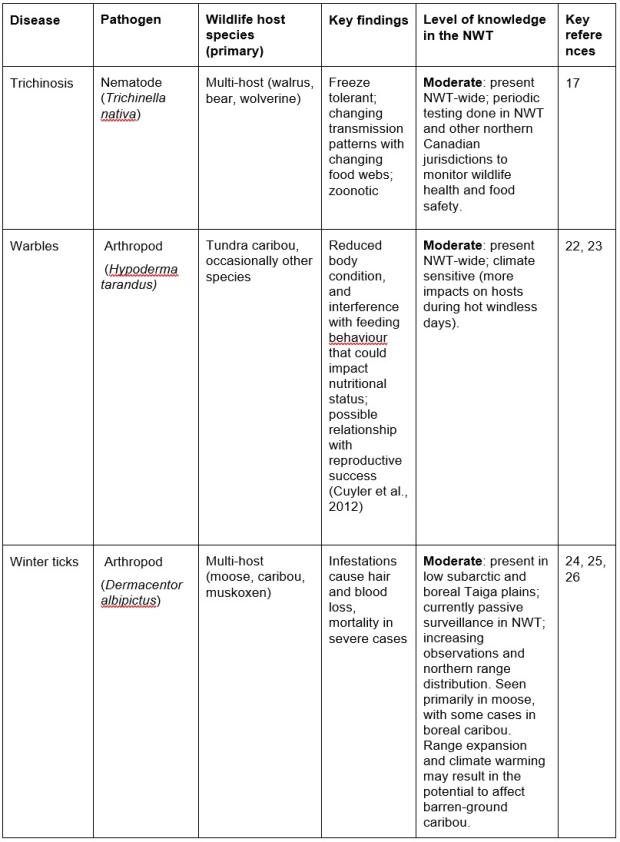
This indicator tracks pathogens with the potential to impact NWT wildlife populations and/or human health (Table 1) and provides a general overview of the available health metrics and how they are measured (see Technical Notes). An exhaustive list of all pathogens that may impact NWT wildlife is beyond the scope of this report, and the status and awareness of diseases, parasites and other stressors impacting wildlife health changes over time. Given these limitations, a number of key wildlife diseases and parasites are regularly tracked in the NWT, and the level of knowledge for each of these indicators is tracked (low, medium, high) and documented below. Similarly, a comprehensive report on wildlife health status for all NWT species is beyond the scope of this indicator, but some key wildlife health concepts are discussed below.
Table 1. Important diseases, the pathogen causing the disease, known wildlife host(s) with key findings of wildlife health effects, and level of knowledge about presence and effects on wildlife determined from monitoring and research. Diseases potentially transmissible to humans are noted as “zoonotic” in key findings.



Tracking wildlife health in large and remote areas is challenging, and benefits from multiple different monitoring approaches. In addition to conventional wildlife health monitoring methods, several innovative approaches have been used to study wildlife health in the NWT and other areas northern Canada.
Local and Traditional Indigenous knowledge
Detection, reporting and sampling of sick or dead wildlife is an important part of a coordinated approach to wildlife health monitoring. In remote regions of the NWT, active and enhanced passive surveillance for the presence of pathogens, disease cases, mortality events, and emerging diseases is augmented by community-based monitoring, reports, and sample collection for laboratory testing (Ref. 3). Interviews with harvesters and land users have provided additional critical information on disease occurrence, cases and mortality events including detailed descriptions of the spatial, annual, and seasonal patterns of morbidities and mortality (Ref. 2). For example, less than 10% of acute mortalities of muskox observed by local hunters were officially reported through other monitoring approaches (Figure 1). This highlights the value of a system to capture reports and samples from local hunters and communities and to increase public communication and outreach on the importance of this information.

Local and Indigenous Knowledge, including Traditional Knowledge provides valuable insights and an understanding of wildlife health that spans multiple generations, which helps inform overall efforts to detect and document trends and drivers in wildlife health. Knowledge from local hunters and Elders spans decades and can describe caribou population trends extending further back in time than the scientific record. Work conducted by Tomaselli et al. illustrated how local and Traditional knowledge can be documented in a way that is readily interpreted and incorporated into status assessments (Ref. 4).

Climate-sensitive parasites as early-warning signs of ecosystem changes
Many pathogens may be sensitive to climate change given that key stages of their life cycle, including development and survival rates in the environment or in their hosts, can be linked to temperature and humidity. With warming temperatures, parasites may expand their range to new locations and potentially infect new host species. Monitoring climate-sensitive pathogens allows us to identify new threats to wildlife and human health while providing a metric to track the biological effects of climate change.
One example is the muskox lungworm Umingmakstrongylus pallikuukensis (Up), which is a well-studied nematode parasite that relies on an ectothermic intermediate host (Deroceras laeve, a slug) to complete its lifecycle. The development rate in the slug intermediate host depends on temperature and occurs more rapidly at warmer temperatures. The lungworm has expanded its previously known range to the north and east, including movement onto Victoria Island (NU), during the last 20 years. The intermediate host slug is also present on adjacent Banks Island, NT (Ref. 29). Under current climate-warming scenarios, it is anticipated that the lungworm could further expand its range and establish on Banks Island, NT, assuming movement of infected muskoxen from Victoria Island to Banks Island (Figure 3. Ref. 28, 31).

Monitoring zoonotic diseases at the human-wildlife interface
Many wildlife species can be reservoirs of pathogens that can infect people (i.e., zoonotic diseases) or domestic animals, including livestock and pets (Ref. 31). A One Health approach aims to optimize health outcomes for wildlife, domestic animals, humans, and the environment by taking a collaborative approach across multiple disciplines.
As an example, the rabies virus is endemic in Arctic foxes throughout a vast area that covers much of the tundra in northern Canada. The virus can affect any mammalian species, is transmitted through the bite of an infected animal, and is invariably fatal. Rabies epidemics appear to fluctuate with Arctic fox abundance (Ref. 20). Cases are seen periodically in northern dog populations, often resulting from spillover from foxes during epidemic years. Dog populations in northern remote communities are often housed outdoors, may not have regular access to rabies vaccinations, and can be exposed to rabies through contact with infected foxes. Cases in dogs can result in potential exposure to their owners and other people and dogs in the community.
In late 2020 and early 2021, the NWT saw an increase in rabies cases in Arctic and red foxes in the Beaufort Delta region of the NWT. Numerous dogs were exposed to and infected with rabies and public health measures were taken to prevent human exposure. The spread of rabies from endemic areas in northern Canada can also occur when infected dogs are inadvertently relocated to areas outside of the rabies endemic area (Ref. 21).
Ongoing targeted and passive monitoring for rabies is done for wildlife and domestic animals across the NWT. Rabies management is done primarily through a focus on surveillance, early detection and preventive measures to prevent domestic animal and human exposure including the regular vaccination of domestic dogs. There is some evidence that trap-vaccinate-release and oral vaccine baits may be effective at controlling rabies prevalence in wild raccoons in other areas, although such programs have not been tested in an Arctic context where there are a number of logistical challenges and different ecological factors to consider (Ref. 32, 33).
Cleaning protocols and education programs can help people reduce the spread of wildlife pathogens
Many wildlife diseases and parasites do not present a risk to human health, but can have important impacts on wildlife health, management and conservation.
For example, the highly transmissible fungus (Pseudogymnoascus destructans) that causes White- Nose Syndrome in bats (Ref 38, 39) can be transported to new areas by contaminated clothes or equipment. Movement of captive or free-ranging wildlife and wildlife parts can also introduce pathogens to new regions.
It is important to avoid handling wildlife directly, and to disinfect personal gear and equipment used in areas where wildlife diseases might be present. Be aware of the rules and best practices if you are handling or translocating wildlife or wildlife parts to minimize the risk of contracting or inadvertently spreading infectious diseases (Ref. 53, 54).
Looking around
There are several key pathogens in southern wildlife populations that have significant impacts on wildlife health and population viability. While these diseases do not currently occur in the NWT, their potential introduction could have significant impacts on wildlife management and conservation in the NWT.
Chronic Wasting Disease
Chronic Wasting Disease (CWD) is a fatal neurodegenerative disease caused by a prion (abnormal protein) that is found in a number of free-ranging ungulate species and populations in North America (Ref. 35). The distribution of CWD in Canada has been limited to Alberta and northern Saskatchewan (see map below) and has spread slowly into historical boreal caribou range in mid-Saskatchewan. In November 2021, CWD was detected in a symptomatic mule deer in Manitoba, close to the western border shared with Saskatchewan. Caribou are susceptible to CWD transmission (Ref. 36) and could be impacted if CWD is introduced to the NWT. Although there is no evidence of this disease affecting people, its introduction could impact public perceptions of food safety. Monitoring is done for CWD in the NWT, with a focus on the southern parts of the territory, and a number of disease control measures are in place to prevent introduction of the disease into the NWT. These include regulations around the movement of live or dead cervids (or cervid parts) from jurisdictions where CWD is present (faq_chronic_wasting_disease_march_2019_en.pdf (gov.nt.ca)).

White-nose syndrome
White-nose syndrome (WNS) is an introduced fungal infection caused by Pseudogymnoascus destructans that has killed millions of bats in eastern North America (Ref. 38, 39). Analysis of patterns of spread suggest that P. destructans will reach all Canadian hibernacula (winter bat habitat) within the next decade (Ref. 39), including northern latitudes. In 2021, WNS was detected for the first time in the province of Saskatchewan, and cases in western Manitoba were documented as the northern-most detections of WNS to date. At least two bat species in the NWT, the little brown myotis (Myotis lucifugus) and northern myotis (M. septentrionalis), are known to be highly susceptible to the fungus.
Avian Influenza
Migratory birds that nest in the Arctic are important carriers of avian influenza viruses, typically caused by influenza A. Although it is often a sub-clinical infection in wild reservoir waterfowl populations, spread and infection in domestic poultry flocks can be severe (Ref. 40) and international surveillance along migratory routes is warranted. Avian influenza is a zoonotic disease which can infect any species of bird, and less commonly mammals. In 2022, a Highly Pathogenic Avian Influenza strain (H5N1) has been detected across Canada in wild birds (including migratory waterfowl and raptors) and in domestic poultry flocks in most Canadian provinces, associated with significant morbidity and mortality. Regular monitoring for avian influenza takes place at select sites along migratory bird flyways in the NWT.
Meningeal Worm
The meningeal worm (P. tenuis) is a parasitic nematode of white-tailed deer common in eastern and central North America (Ref. 41). Its range has progressively expanded north and west across Saskatchewan and into the boreal forest fringe. Land use change and climate change are expected to facilitate further expansion with its white-tailed deer host (Ref. 42). While its impacts on white-tailed deer are negligible, it is highly pathogenic for caribou, moose, mule deer and elk, typically causing fatal neurological disease.
Lice in canids
In Alaska, the louse Trichodectes canis, thought to have originated in domestic dogs, has spilled over into wolves. In wolves, this louse causes significant lesions, poor condition and reduced survival, and may adversely affect the trapping industry. To date, this louse species has not been documented in wild canids in northern Canada, however, it is a potentially invasive species of concern that could affect wild animal health.
Looking forward
Northern ecosystems are experiencing unprecedented change with global climate warming and an increasing human footprint. These changes will affect wildlife health in a myriad of ways, with high uncertainty in the net outcome.
Proactive health surveillance systems that rely on different and complementary methods and types of knowledge documentation– including hunter-based sampling, documenting scientific, local and Traditional Indigenous knowledge, passive and active surveillance, and targeted research – are critical to detect and respond to emerging threats. For example, community-based monitoring, including hunter-based sampling, of caribou and many other species in the NWT, has provided information on wildlife health that would otherwise be challenging and expensive to obtain (Ref. 30). The engagement of local harvesters in sample collection from animals harvested for subsistence provides the opportunity to more-regularly monitor health indicators (e.g., stress, disease, condition, nutrition) and increase sample sizes for better population estimates. Together with samples from wildlife handled for research and monitoring purposes (including collaring), these can provide ongoing health tracking at a temporal resolution that would not be possible for conventional monitoring alone. Early detection of emerging pathogens and changes in wildlife health enables pro-active management that pre-empt wildlife population declines, as well as timely implementation of necessary public health measures, to ensure wildlife sustainability, food security and human health, and protect domestic animals and an emergent northern livestock industry.
Find out more
Canadian Wildlife Health Cooperative - http://www.cwhc-rcsf.ca/
Field Guide to Common Wildlife Diseases and Parasites in the NWT: https://www.ecc.gov.nt.ca/sites/ecc/files/field_guide_wildlife_diseases.pdf.
ECC Wildlife Diseases: https://www.ecc.gov.nt.ca/en/services/wildlife-diseases
Technical Notes
Selected wildlife health metrics of individual animal health, what they mean, why they are monitored and how they can be measured or sampled are provided in the Table below.



References
Ref.1. Stephen, C. 2014. Toward a Modernized Definition of Wildlife Health. J. Wildl. Dis. 50: 427–430.
Ref. 2. Tomaselli, M., Kutz, S., Gerlach, C. & Checkley, S. 2018. Local knowledge to enhance wildlife population health surveillance: Conserving muskoxen and caribou in the Canadian Arctic. Biol. Conserv. 217: 337–348, Available at https://www.sciencedirect.com/journal/biological-conservation.
Ref. 3. Kutz, S. and Tomaselli, M. 2019. “Two-eyed seeing” supports wildlife health. Science 364: 1135 LP – 1137.
Ref. 4. Peacock, S. J. et al. 2020. Linking co-monitoring to co-management: Bringing together local, traditional, scientific knowledge in a wildlife status and assessment framework. Arctic. Science. 6: 247–266.
Ref. 5. Conservation of Arctic Flora and Fauna (CAFF). 2013. Arctic Biodiversity Assessment 2013. Available at http://www.arcticbiodiversity.is
Ref. 6. Kutz, S. J., Hoberg, E. P., Polley, L. & Jenkins, E. J. 2005. Global warming is changing the dynamics of Arctic host-parasite systems. Proc. R. Soc. B Biol. Sci. 272 : 2571–2576.
Ref. 7. Kutz, S. J. et al. 2013. Invasion, establishment, and range expansion of two parasitic nematodes in the Canadian Arctic. Global Change Biol. 19: 3254–3262.
Ref. 8. Carlsson, A. M., Dobson, A. & Kutz, S. J. 2019. The Impact of Infectious Agents on Rangifer Populations. In Reindeer Caribou p. 315–352, doi:10.1201/9780429489617-10.
Ref. 9. Dragon, D. C., Elkin, B. T., Nishi, J. S. & Ellsworth, T. R. 1999. A review of anthrax in Canada and implications for research on the disease in northern bison. J. Appl. Microbiol. 87: 208–213.
Ref. 10. Tessaro, S. V., Gates, C. C. & Forbes, L. B. 1993. The brucellosis and tuberculosis status of Wood Bison in the Mackenzie Bison Sanctuary, Northwest Territories, Canada. Can. J. Vet. Res. 57: 231–235.
Ref. 11. Wobeser, G. 2009. Bovine tuberculosis in Canadian wildlife: An updated history. Can. Vet. J. 50, 1169–1176.
Ref. 12. Forbes, L. B. 1991.Isolates of Brucella suis biovar 4 from animals and humans in Canada, 1982-1990. 32: 3.
Ref. 13. Tomaselli, M. et al. 2019. A transdisciplinary approach to Brucella in muskoxen of the western Canadian Arctic 1989–2016. EcoHealth doi:10.1007/s10393-019-01433-3.
Ref. 14. Mavrot, F. et al. 2020. Novel insights into serodiagnosis and epidemiology of Erysipelothrix rhusiopathiae, a newly recognized pathogen in muskoxen (Ovibos moschatus). PLoS ONE 15: 1–14.
Ref. 15. Kutz, S. et al. 2015. Erysipelothrix rhusiopathiae associated with recent widespread muskox mortalities in the Canadian Arctic. Can. Vet. J. 56: 560–563.
Ref. 16. Forde, T. et al. 2016. Genomic analysis of the multi-host pathogen Erysipelothrix rhusiopathiae reveals extensive recombination as well as the existence of three generalist clades with wide geographic distribution. BMC Genomics 17: 461.
Ref. 17. Jenkins, E. J. et al. 2013. Tradition and transition: parasitic zoonoses of people and animals in Alaska, Northern Canada, and Greenland. in Advances in Parasitology vol. 82: 33–204 (Elsevier)
Ref. 18. Duignan, P. J. et al. 1997. Epizootiology of morbillivirus infection in harp, hooded, and ringed seals from the Canadian Arctic and western Atlantic. J. Wildl. Dis. 33: 7–19.
Ref. 19. VanWormer, E. et al. 2019. Viral emergence in marine mammals in the North Pacific may be linked to Arctic sea ice reduction. Sci. Rep. 9: 15569.
Ref. 20. Simon, A. et al. Dynamics and persistence of rabies in the Arctic. Polar Res. (2019) doi:10.33265/polar.v38.3366.
Ref. 21. Curry, P. et al. 2016. Translocated dogs from Nunavut and the spread of rabies. Can. Commun. Dis. Rep. 42: 121–124.
Ref.22. Thomas, D. D. & Kiliaan, H. P. L. 1990. Warble infestations in some Canadian caribou and their significance. Rangifer 10: 409.
Ref. 23. Weladji, R. B., Holand, Ø. & Almøy, T. 2003. Use of climatic data to assess the effect of insect harassment on the autumn weight of reindeer (Rangifer tarandus) calves: Effect of insect harassment on the productivity of Rangifer tarandus. J. Zool. 260: 79–85.
Ref. 24. Samuel, W. M. 1989. Locations of moose in northwestern Canada with hair loss probably caused by the winter tick, Dermacentor albipictus (Acari: lxodidae). J. Wildl. Dis. 25: 436–439 .
Ref. 25. Chenery, E. S., Harms, N. J., Mandrak, N. E. & Molnár, P. K. 2020. First records of Dermacentor albipictus larvae collected by flagging in Yukon, Canada. Parasit. Vectors 13, 565 (2020).
Ref. 26. Kashivakura, C. K. 2013. Detecting Dermacentor albipictus, the winter tick, at the northern extent of its distribution range: Hunter-based monitoring and serological assay development. University of Calgary.
Ref. 27. Mavrot, F., Rothenburger, J. L. & Kutz, S. 2021. Wildlife health and a growing agriculture industry in the Northwest Territories: A report on active and passive northern wildlife health surveillance activities done through the University of Calgary. 128+vi.
Ref. 28. Kafle, P. et al. 2020. Range expansion of muskox lungworms track rapid arctic warming: implications for geographic colonization under climate forcing. Sci. Rep. 1–14 doi:10.1038/s41598-020-74358-5.
Ref. 29. iNaturalist.2018. iNaturalist report of the Meadow slug (Deroceras laeve) near Sachs Harbour, Banks Island, NT. https://www.inaturalist.org/observations/15662363.
Ref. 30. Carlsson, A. M., Veitch, A. M., Popko, R. A., Behrens, S. & Kutz, S. J. 2016. Monitoring wildlife health for conservation and food security in the Canadian north: a case study from the Sahtu settlement area in the Northwest Territories. in One Health Case Studies: Addressing Complex Problems in a Changing World (eds. Cork, S. C., Hall, D. C. & Lijebjelke, K. A.) 132–151 pp. 5M Publishing Ltd.
Ref. 31. Daszak, P., Cunningham, A. & Hyatt, A. 2020. Emerging Infectious Diseases of Wildlife - Threats to Biodiversity and Human Health. Science 287: 443.
Ref. 32. Gilbert, A. et al. 2018. Efficacy of Ontario rabies vaccine baits (ONRAB) against rabies infection in raccoons. Vaccine 36: 4919–4926.
Ref. 33. Rosatte, R. C. et al. 2009. The control of raccoon rabies in Ontario Canada: proactive and reactive tactics 1994–2007. J. Wildl. Dis. 45: 772–784.
Ref. 34. Buhler, K. J. 2020. Hopping species and borders: detection of Bartonella spp. in avian nest fleas and arctic foxes from Nunavut, Canada. Parasites and Vectors 13: 469.
Ref. 35. Mitchell, G. B. et al. 2012. Experimental oral transmission of chronic wasting disease to reindeer (Rangifer tarandus tarandus). PLoS ONE 7, e39055 (2012).
Ref. 36. Benestad, S. L., Mitchell, G., Simmons, M., Ytrehus, B. & Vikøren, T. 2016. First case of chronic wasting disease in Europe in a Norwegian free-ranging reindeer. Vet. Res. 47: 1–7.
Ref. 37. Environment and Climate Change. NWT. 2020. Chronic wasting disease in the NWT. https://www.ecc.gov.nt.ca/sites/ecc/files/resources/fact_sheet_-_chronic....
Ref. 38. Fenton, M. B. 2012. Bats and white-nose syndrome. Proc. Natl. Acad. Sci. 109: 6794–6795.
Ref. 39. Hoyt, J. R., Kilpatrick, A. M. & Langwig, K. E. 2021. Ecology and impacts of white-nose syndrome on bats. Nat. Rev. Microbiol. 19: 196–210.
Ref. 40. Bradley, M. J., Kutz, S. J., Jenkins, E. & O’Hara, T. M. 2005. The potential impact of climate change on infectious diseases of Arctic fauna. Int. J. Circumpolar Health 64: 468–477.
Ref. 41. Wasel, S. M., Samuel, W. M. & Crichton, V. 2003. Distribution and ecology of meningeal worm, Parelphostrongylus tenuis (Nematoma), in northcentral North America. J. Wildl. Dis. 39: 338–346.
Ref. 42. Pickles, R. S. A., Thornton, D., Feldman, R., Marques, A. & Murray, D. L. 2013. Predicting shifts in parasite distribution with climate change: a multitrophic level approach. Glob. Change Biol. 19: 2645–2654.
Ref. 43. Beaulieu, M. & Costantini, D. 2014. Biomarkers of oxidative status: missing tools in conservation physiology. Conserv. Physiol. 2: cou014–cou014.
Ref. 44. Elkin, B., T. Armstrong and T. Epp. 2017. Anthrax in the Mackenzie wood bison (Bison bison athabascae) population: 2012 anthrax outbreak and historical exposure in non outbreak years. Journal of Wildlife Diseases 53: 769-780.
Ref. 45. Salb, A., C. Stephen, C. Ribble and B. Elkin. 2014. Descriptive Epidemiology of detected anthrax outbreaks in wild wood bison (Bison bison athabascae) in northern Canada from 1962-2008. Journal of Wildlife Diseases 50(3): 459-468.
Ref. 46. Nishi, J.S., D.C. Dragon, B.T. Elkin, J. Mitchell, T.R. Ellsworth and M.E. Hugh-Jones. 2002. Emergency response planning for anthrax outbreaks in bison herds of Northern Canada: A balance between policy and science. Annals of the New York Academy of Sciences 969:245-250.
Ref. 47. Gates, C.C., B.T. Elkin and D. Dragon. 2001. Anthrax. In: Infectious diseases of wild mammals, Third Edition. eds. E.S. Williams and I.K. Barker, p. 396-412. Iowa State University Press. Ames, Iowa.
Ref. 48. Gates, C.C., B.T. Elkin and D.C. Dragon. 1995. Investigation, control and epizootiology of anthrax in a geographically isolated, free-roaming bison population in northern Canada. Canadian Journal of Veterinary Research 59:256-264.
Ref. 49. Nishi, J.S., C. Stephen and B.T. Elkin. 2002. Implications of agricultural and wildlife policy on management and eradication of bovine tuberculosis and brucellosis in free-ranging wood bison of northern Canada. Annals of the New York Academy of Sciences 969:236-244.
Ref. 50. Shury, T.K., J.S. Nishi, B.T. Elkin and G.A. Wobeser. 2015. Tuberculosis and brucellosis in wood bison (Bison bison athabascae) in northern Canada: a renewed need to develop options for future management. Journal of Wildlife Diseases 51:543-554.
Ref. 51. Nishi, J.S., T. Shury and B.T. Elkin. 2006. Wildlife reservoirs for bovine tuberculosis (Mycobacterium bovis) in Canada: Strategies for management and research. Veterinary Microbiology 112:325-338.
Ref. 52. Dragon, D.C. and B.T. Elkin. 2001. An overview of early anthrax outbreaks in northern Canada: field reports of the Health of Animals Branch, Agriculture Canada, 1962-71. Arctic 54:32-40.
Ref. 53. Jakob-Hoff R.M., MacDiarmid S.C., Lees C., Miller P.S., Travis D. & Kock R. 2014. Manual of Procedures for Wildlife Disease Risk Analysis. World Organization for Animal Health, Paris, 160 pp. Published in association with the International Union for Conservation of Nature and the Species Survival Commission. Available at http://www.cbsg.org/sites/cbsg.org/files/documents/IUCN%20Wildlife%20DRA%20Manual%20PUBLISHED%202014_0.pdf
Ref. 54. Hartley and Sainsbury. 2017. Methods of Disease Risk Analysis in Wildlife Translocations for Conservation Purposes. Ecohealth 14(Suppl 1): 16–29.
15.6 Status of wildlife pathogens in aquatic environments
This indicator provides an overview of some important pathogens of aquatic vertebrate species in the NWT. It is not possible to include all pathogens of aquatic vertebrates in this chapter. We highlight pathogens that reflect a diversity of affected host species and ecosystems. Further, the pathogens highlighted here share the key characteristics of infecting multiple host species and exhibiting expanding geographic distributions. We focus on pathogens affecting freshwater vertebrates but also briefly discuss two important pathogens in marine mammals that may be present in the NWT.

Freshwater ecosystems are threatened on a global scale, with infectious diseases being major contributing factors in the declines of freshwater vertebrates (Ref. 1-3). Modeling of climate change induced changes in host and pathogen systems in northern ecosystems indicate the potential for severe outcomes for native populations (Ref. 4-6), making it imperative to create predictive models of northern host–pathogen systems and to conduct broad-scale surveillance studies (Ref. 5, 7-10).
An overview of how wildlife health is defined in this report as well the importance of monitoring wildlife health appears in Chapter 15.5 Status of Wildlife Health in Terrestrial Environments. Of key importance is that the definition of wildlife health recognizes that health is not merely the ‘absence of disease’ but depends on multiple interacting biological, social, and environmental factors (Ref. 11).
The indicator was prepared by contractors with extensive experience in amphibian studies in the NWT (Orchis Environmental, ON and Palustris Environmental, AB, and by subject experts from University of Calgary, and was reviewed by the Government of the Northwest Territories, Department of Environment and Climate Change. The information presented in this indicator is a compilation of work done by the Government of the NWT (GNWT), wildlife co-management partners, academia, communities, and individuals conducting research and monitoring in the NWT.
Current View: status and trend
This indicator tracks pathogens with the potential to impact aquatic vertebrate wildlife in the NWT. Table 1 provides a general overview of several pathogens known to occur in the NWT and the level of certainty regarding how these pathogens affect aquatic wildlife in the NWT. Additional information on wildlife pathogens follows Table 1.
Table 1. Diseases, associated pathogen, known host(s), key findings of health effects, and level of knowledge about presence and effects on aquatic wildlife determined from monitoring and research. Diseases potentially transmissible to humans are noted as “zoonotic” in key findings.
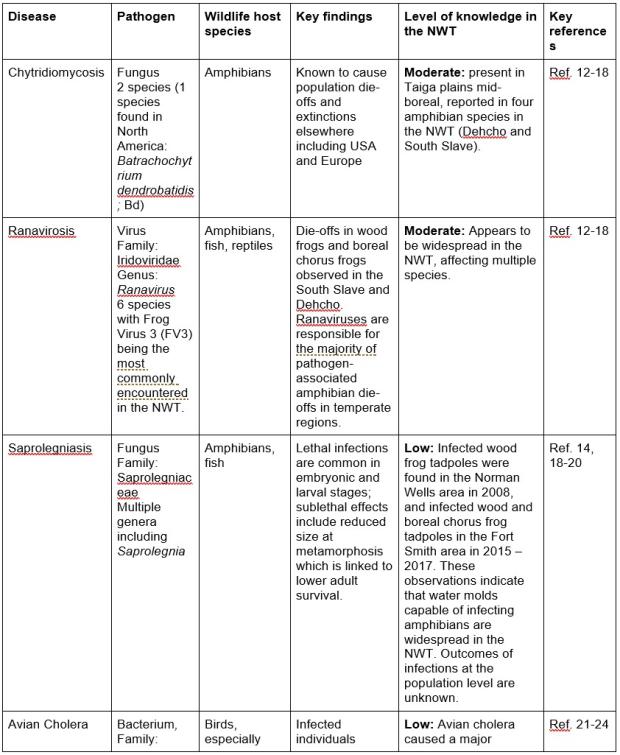
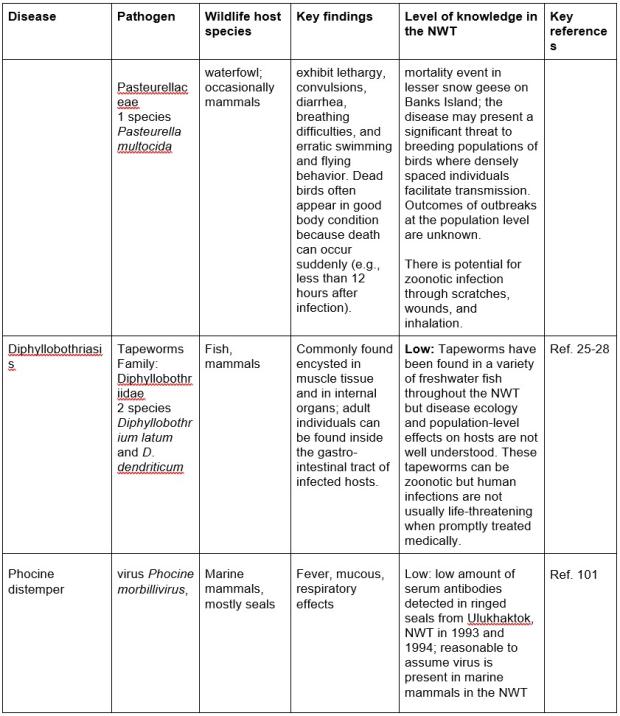
Amphibian pathogens
The health of amphibian populations can serve as an indicator of ecosystem change and overall ecosystem health (reviewed by Ref. 29). Most individuals of most amphibian species spend their entire lives within one km of their natal ponds (Ref. 30) and therefore their health can serve as an indicator of the local environment. Healthy communities of amphibians require intact habitats with sufficient complexity to accommodate the diverse requirements of different species (Ref. 12). Local elders in Fort Fitzgerald, Fort Smith, and Fort Resolution provided valuable knowledge on local amphibian populations and reported a steady decline in amphibian calling activities in the area over the past 20-30 years (Ref. 14).
Two amphibian pathogens implicated in global amphibian declines have been detected in the NWT: The chytrid fungus Batrachochytrium dendrobatidis (Bd) and ranaviruses (Ref. 13, 31, 32). Diseases associated with these pathogens are listed as notifiable by the World Organisation for Animal Health (Ref. 33).
Chytrid fungi
Two species of chytrid fungi, Batrachochytrium dendrobatidis (Bd) and B. salamandrivorans (BSal), are known to infect amphibians, potentially causing the disease chytridiomycosis, but to date only Bd has been found in North America (Ref. 32). Chytridiomycosis is associated with declines in at least 500 amphibian species worldwide, including extirpations and extinctions (Ref. 32, 34). Infected adult individuals often exhibit morbidity, as well as irregular skin thickening and sloughing, potentially disrupting osmotic processes (Ref. 35). In tadpoles, the fungus predominately attacks the keratinized mouthparts (Ref. 35). Ultimately, infected individuals often succumb to anorexia and/or cardiac arrest (Ref. 35).
Bd was first detected in the NWT in wood frogs (Rana sylvatica = Lithobates sylvaticus), boreal chorus frogs (Pseudacris maculata), and boreal toads (Anaxyrus boreas) in the Dehcho in 2007-2008 (Ref. 13). Amphibian health surveys conducted in the Sahtu in 2007-2008 did not detect Bd infected individuals in the region but the Sahtu has not been re-assessed since 2008. Studies in Wood Buffalo National Park (WBNP) and adjacent areas in 2009-2012 and 2015-2017, confirmed a restricted distribution and low prevalence of Bd in wood frogs and boreal chorus frogs in the South Slave region (Ref. 14). Bd was also detected in WBNP in 2018 in water samples that were assessed using eDNA techniques.
No amphibian die-offs attributable to Bd have been documented in the NWT. This is consistent with findings of Bd in amphibian populations elsewhere in Canada (e.g., Ref. 36, 37). However, sublethal chytrid infections can have negative effects on the overall fitness of the infected individuals (e.g., Ref. 38, 39) and are therefore an ongoing potential threat to amphibians in the NWT.
Ranaviruses
Ranavirus is a genus of viruses within the family Iridoviridae whose members infect fish, reptiles, and amphibians (reviewed by Ref. 40). Although ranaviruses have been found in more than 100 amphibian species worldwide, a lack of large-scale studies on host and geographic range suggests that the prevalence, and therefore importance of ranaviruses in amphibian health, is likely underestimated (Ref. 31, 40). Ranavirus infections can cause a potentially lethal systemic disease including anorexia, organ necrosis, and severe hemorrhaging in the extremities (Ref. 41). Sublethal effects of infection can also occur and are an area of active research because of the potential for long-reaching effects on population health (e.g., Ref. 42, 43).
There is accumulating evidence that ranaviruses are persistent, widespread pathogens in the NWT. Ranaviruses were first documented in wood frogs in the Dehcho and Sahtu regions in 2007-2008 (Ref. 17) and in the South Slave in 2009 – 2012 (Ref. 14). Amphibian surveys in the Fort Liard area (Dehcho) in 2019 encountered wood frog mortalities which were subsequently confirmed to be caused by a ranavirus outbreak (Ref. 15). Field studies conducted between 2015 – 2017 focused on the epidemiology and evolution of amphibian ranaviruses in the South Slave region (Ref. 16, 44-46). Ranavirus was widespread across the region, with wood and boreal chorus frogs exhibiting high infection rates, whereas Canadian toads exhibited low infection rates. Prevalence and viral loads in tadpoles were generally higher compared to terrestrial individuals, and the majority of post-metamorphic individuals that tested positive for ranavirus showed few, if any, signs of disease, suggesting their possible role as a reservoir in this system (Ref. 16). This hypothesis was supported in an experimental exposure study involving post-metamorphic wood frogs raised from eggs collected in the NWT, with the majority of exposed individuals either sustaining a sublethal infection or clearing all detectable virus particles (Ref. 47). Genetic analyses of ranaviruses collected in the South Slave revealed at least two distinct ranavirus species (Ref. 45, 46), and spatio-temporal analysis of phylogenetic data for amphibian ranaviruses, which included ranaviruses isolated from amphibians in the NWT, suggests that ranaviruses were introduced into Canadian amphibian populations within the last 100 years, potentially in the mid-1940s (Ref. 46).
Water molds
Infections with other amphibian pathogens, in particular water molds in the genera Achlya and Saprolegnia, are commonly observed in tadpoles in the NWT (Ref. 14). Water mold infections have not been conclusively linked with amphibian declines, but infections in eggs and tadpoles may be under-appreciated causes of mortality in immature amphibians. Lack of attention to this group of aquatic pathogens may be at least partly explained by the conventional wisdom that water mold infections are “opportunistic” – that is, eggs and tadpoles that are already in poor condition for other reasons are more likely to become infected and die. However, laboratory studies demonstrated that Saprolegnia and other water molds can readily cause mortality in healthy amphibian eggs and larvae in a dose-dependent fashion (Ref. 48-50), which is a pattern associated with pathogens that can be primary causes of disease.
Live tadpoles infected with water molds were found in wood frog populations in Norman Wells in 2008 (Ref. 17), and in wood and boreal chorus frog populations in Fort Smith and adjacent WBNP in 2015 – 2017 (Ref. 14). Infected egg masses are commonly found, and as much as 50% of wood frog egg masses at a given breeding site have been found to be infected by water molds in the Lower Athabasca region of northern Alberta and the South Slave region (Ref. 14). It is currently unknown what role(s) water molds play in the persistence and stability of amphibian populations in the boreal forest.

Fish pathogens
There are approximately 50 species of freshwater and anadromous fish species documented in the NWT (Ref. 51). Fish populations in the NWT are generally considered stable and healthy, although some instances of overharvesting have occurred in the past and sustainable management plans are now required (e.g., Ref. 51 - 53). In terms of fish pathogens documented in the NWT, helpful descriptive lists have been produced (e.g., Ref. 54) but in-depth investigations of the effects of pathogens and/or parasites on fish health in the NWT are limited. Further, climate change may lead to the immigration of new species into the NWT, potentially accompanied by new pathogens (Ref. 55-56).
Tapeworms
Two tapeworms in the genus Diphyllobothrium occur in multiple fish species in the NWT, these tapeworms negatively impact harvest value, and they can cause important infections in humans and dogs (Ref. 51, 54, 58, 59). Tapeworms are internal parasites with an indirect life cycle that involves several intermediate hosts before maturing in the final host (Ref. 25-28). While larval stages are commonly found in muscle tissue and in internal organs, adults live and reproduce inside the gastro-intestinal tract of the infected host (Ref. 26). Human infections occur when raw or undercooked fish is consumed but long-term effects on human health are minimized with prompt medical treatment.
Bird pathogens
The NWT is inhabited by more than 270 species of birds, of which 87% are migratory, relying on Arctic and boreal habitats for breeding but overwintering further south (Ref. 51). Migratory waterfowl are important human food sources and important components of northern ecosystems (Ref. 51). As with the other parasites and pathogens mentioned in this report, climate change is likely to affect avian diseases, causing further effects in northern ecosystems including changes in species community composition (Ref. 24, 61, 64-66). Avian cholera is highlighted in this report because of its potential to cause massive die-offs (epizootics) in multiple species of waterfowl in the Canadian north and because of its small but important potential to cause zoonotic disease (Ref. 23, 24, 62, 69).
Avian Cholera
Avian cholera is an infectious disease caused by the bacteria Pasteurella multocida, affecting wild and domestic bird populations (Ref. 23). It is a significant infectious disease of wild waterfowl in North America, but infection outcomes vary greatly across species (Ref. 22). Infected individuals commonly exhibit lethargy, convulsions, diarrhea, breathing difficulties, as well as erratic swimming and flying behavior (Ref. 21, 23). Severe infections are often associated with the sudden death of the bird, and outbreaks can lead to concerns about long-term impacts on the affected waterfowl populations (Ref. 21, 23, 24, 69). Research suggests that infected birds that don’t die may act as reservoirs and carriers of the bacteria, spreading the bacterium across the landscape (Ref. 23, 62, 70). Proper hygiene (e.g., gloves, handwashing) can generally protect humans, but mass deaths of waterfowl can impact populations (Ref. 23).
Infections with avian cholera were first reported in wild waterfowl in the eastern USA in the early 1940s, before spreading throughout North America causing annual outbreaks and epidemics (Ref. 23). The pathogen has been found in domestic birds worldwide but outbreaks in wild birds predominately affect waterfowl in North America (Ref. 23). Massive die-offs in the 1990’s of approximately 50,000 birds on Banks Island were attributed to avian cholera (Ref. 69). Surveillance and predictive modeling are needed to assess the current and lingering effects of avian cholera in wild bird populations in the NWT.
Phocine Distemper
Phocine distemper is a systemic and potentially lethal disease caused by the virus Phocine morbillivirus, which affects a wide range of marine mammals (Ref. 84). Infections are commonly associated with fever, mucous oozing from nostrils and eyes, and severe respiratory effects in marine mammals (Ref. 84). Phocine ddistemper outbreaks caused mortalities in seal populations in Europe, the USA, and eastern Canada, as well as in sea otters in the North Pacific Ocean (Ref. 85). Furthermore, evidence for infection (antibodies to the virus) has been found in Atlantic walruses (Odobenus rosmarus) in Nunavut (Ref. 86, 87). Changes in Arctic sea ice due to climate change may increase the transmission of the pathogen among marine mammals in the Arctic Ocean (Ref. 88). A low amount of serum antibodies detected in ringed seals from Ulukhaktok, NWT in 1993 and 1994 confirmed previous exposure to phocine distemper (Ref 101). It is reasonable to assume that this virus is already present in marine mammals in the NWT because it is a multi-host pathogen that spreads among marine mammal species, and there is evidence of the virus in Alaska and eastern Canadian Arctic (Ref. 88). Understanding the distribution and effects of the pathogen on marine mammals in the NWT will require focused study.
Cleaning protocols and education programs can help people reduce the spread of aquatic wildlife pathogens.
Many pathogens do not present a risk to human health but can severely impact wildlife populations and ecosystem health.
For example, the fungus (Batrachochytrium spp.) that causes chytrid disease in amphibians can be transported to new areas by people’s gear, such as waders and boots. Transportation of infected wildlife by people can introduce pathogens to new regions.
Avoid handling wildlife whenever possible and follow proper protocols for handling and translocating wildlife when handling cannot be avoided.
Precautions should be taken to prevent accidental transfer of pathogens among wetlands and river systems. Practical suggestions for working near wetlands can be found in “Decontamination Protocol for Field Work with Amphibians and Reptiles in Canada”, the “Manual of Procedures for Wildlife Disease Risk Analysis”, and the “Methods of Disease Risk Analysis in Wildlife Translocations for Conservation Purposes” (Ref. 71-73).
Looking around
The following pathogens have not yet been detected in the NWT but may be emerging concerns for northern wildlife populations. This is not an exhaustive list.
Whirling Disease
Whirling disease is a parasitic infection that affects a variety of salmonid fish species including whitefish, rainbow trout, and several species of salmon (Salmonidae). The disease is caused by the parasite Myxobolus cerebralis, which requires an aquatic worm (Tubifex tubifex) for part of its lifecycle and a salmonid fish for the other part of its lifecycle. Severe infections in fish are characterized by skeletal deformities and erratic swimming behavior (“whirling”) caused by damages to the neural system (Ref. 74). Outbreaks can have major economic impacts when they occur in commercial aquaculture facilities but can also have serious ecological impacts in wild populations (Ref. 74, 75). Infections are often fatal in younger fish while adult fish can often sustain sublethal infections (Ref. 76, 77). Members of the genus Oncorhynchus, especially rainbow trout (Oncorhynchus mykiss), seem to be particularly prone to severe infections (Ref. 76). The parasite originated in Europe and was likely introduced into North America in the 1950s (Ref. 78). Since then, whirling disease has been found in 25 US states, including Alaska, and was detected in Alberta in 2016 (Ref. 77, 79, 80). Whirling disease now occurs in several water sheds in Alberta, including the Peace and Athabasca River watersheds (Ref. 77). Due to the occurrence of the parasite in Alberta and Alaska watersheds, it is probable that the pathogen will be introduced to the NWT in the (near) future. Salmonids are ecologically important and are also an important food source for many people in the NWT. Salmonids appear to be changing in distribution and abundance in the NWT, as reflected by the increasing diversity and number of fish harvested in the NWT (Ref. 55). As such, whirling disease has the potential to become a significant parasite in the NWT.
Avian Influenza
Avian Influenza is a contagious viral infection caused by various Influenza A viruses that can cause serious disease in domestic and wild bird species (Ref. 67). Migratory waterfowl and shorebirds infected with this disease are often able to spread the disease, and infections can range from asymptomatic to severe (Ref. 67, 81, 82). In severe infections, birds often show neurological, digestive, and respiratory problems leading to multi-organ failure and death (Ref. 67). Outbreaks in commercial poultry operations can result in large economic losses (Ref. 81, 83). Although avian influenza viruses do not commonly infect mammals, there is potential for transmission, and infections with avian influenza viruses in humans have been documented (Ref. 67, 68). The pathogen has not been detected in the NWT yet, but migratory waterfowl and shorebirds in the NWT are likely carriers of avian influenza viruses with the potential to spread the pathogen (Ref. 4). Limited monitoring along migratory bird flyways has been conducted in the NWT, but more research and surveillance are needed to assess risk and address management strategies.
Brucellosis in marine mammals
Brucellosis is a disease caused by Gram negative bacteria in the genus Brucella spp. Although these bacteria are most known for their ability to infect and cause disease in terrestrial mammals (see for example 15.5. Status of wildlife health in terrestrial environments), two species, B. ceti and B. pinnipedialis, have been identified in marine mammals worldwide (Ref. 89-91). Clinical signs include skin abscesses, meningoencephalitis, neonatal mortality, and miscarriage (Ref. 87). Both species of marine brucellosis have been isolated from marine mammals across the North American coast, including the Canadian Arctic (Ref. 92-95). Therefore, it is expected that the pathogen is present in marine mammals in the NWT. Like the terrestrial strains, the marine species can also cause disease in humans, and as of 2018, four cases of B. ceti infections have been reported in humans (Ref. 96, 97). It is of concern that traditionally harvested Atlantic walrus (Odobenus rosmarus) and ringed seals (Phoca hispida) in the eastern Canadian Arctic were found to be seropositive for the pathogen (Ref. 92).
Ranavirus in reptiles and fish
Several ranaviruses that affect amphibians are also capable of infecting and causing disease in fish and reptiles (e.g., Ref. 2, 57, 98-100). It is reasonable to assume that there is potential for transmission to amphibians, reptiles, and fish in the NWT. Red-sided garter snakes (Thamnophis sirtalis) in the South Slave region have been observed feeding on wood frogs (Ref. 14), which is one possible mechanism of transmission among amphibians and reptiles within the food webs of the South Slave region. To examine this possibility, several road-killed red-sided garter snakes were collected in Wood Buffalo National Park 2015 -2017 and tested for ranavirus infection (Ref. 14). Although all snake carcasses tested negative for ranavirus, more sampling is needed. Further investigation of links between the health of amphibians and reptiles in the NWT should consider the potential for shared pathogens like ranaviruses.
Looking forward
Early detection of emerging pathogens and changes in wildlife health should enable pro-active management that can prevent wildlife population declines.
Find out more
Canadian Wildlife Health Cooperative:
NWT Wildlife Harvesters Guide:
https://www.ecc.gov.nt.ca/sites/ecc/files/field_guide_wildlife_diseases.pdf
References
Ref. 1. Cunningham, A. A. 2018. Infectious disease threats to amphibian conservation. In The Glasgow Naturalist (Vol. 27). Glasgow Natural History Society.
Ref. 2. Bienentreu, J. F., and Lesbarrères, D. 2020. Amphibian disease ecology: Are we just scratching the surface? Herpetologica, 76(2), 153–166.
Ref. 3. Secretariat of the Convention on Biological Diversity 2020. Global Biodiversity Outlook 5. United Nations, Montréal, 211 pp.
Ref. 4. Bradley MJ, Kutz SJ, Jenkins E, and O'Hara TM. 2005. The potential impact of climate change on infectious diseases of Arctic fauna. Int J Circumpol Health 64: 468–77.
Ref. 5. Burek KA, Gulland FM, and O'Hara TM. 2008. Effects of climate change on Arctic marine mammal health. Ecol Appl 18: 126–34.
Ref. 6. Hueffer K, O'Hara TM, and Follmann EH. 2011. Adaptation of mammalian host-pathogen interactions in a changing Arctic environment. Acta Vet Scand 53: 17.
Ref. 7. Kutz SJ, Jenkins EJ, Veitch AM, et al. 2009. The Arctic as a model for anticipating, preventing, and mitigating climate change impacts on host–parasite interactions. Vet Parasitol 163: 217–28.
Ref. 8. Davidson R, Simard M, Kutz SJ, et al 2011. Arctic parasitology: Why should we care? Trends Parasitol 27: 239–45.
Ref. 9. Hoberg EP, Polley L, Jenkins EJ, et al 2008. Integrated approaches and empirical models for investigation of parasitic diseases in northern wildlife. Emerg Infect Dis 14: 10–17.
Ref. 10. Revich B, Tokarevich N, and Parkinson AJ. 2012. Climate change and zoonotic infections in the Russian Arctic. Int J Circumpolar Health 71; doi:10.3402/ijch.v71i0.18792.
Ref. 11. Stephen, C. 2014. Toward a Modernized Definition of Wildlife Health. J. Wildl. Dis. 50: 427–430.
Ref. 12. ECC: Environment and Climate Change 2017. Management Plan for Amphibians in the Northwest Territories. Species at Risk (NWT) Act Management Plan and Recovery Strategy Series. Environment and Climate Change, Government of the Northwest Territories, Yellowknife, NT. 73 + iii pp.
Ref. 13. Schock, D. M., Ruthig, G. R., Collins, J. P., Kutz, S. J., Carrière, S., Gau, R. J., Veitch, A.M., Larter, N.C., Tate, D.P., Guthrie, G. and Allaire, D. G. 2010. Amphibian chytrid fungus and ranaviruses in the Northwest Territories, Canada. Diseases of Aquatic Organisms, 92(2-3), 231–240.
Ref. 14. Bienentreu JF and Schock DM, unpubl. data and pers. comm.
Ref. 15. Dulisse, J. 2019 Amphibian Surveys, Fort Liard/Dehcho Region, Northwest Territories.
Ref. 16. Bienentreu JF, Schock DM, Greer AL, Lesbarrères D. 2022. Ranavirus amplification in low-diversity amphibian communities. Frontiers in Veterinary Science, special issue Emerging Infections and Diseases of Herpetofauna. 9:755426. DOI: 10.3389/fvets.2022.755426
Ref. 17. Schock, D. M. 2009. Amphibian Population and Pathogen Surveys in the Dehcho and Sahtu, Northwest Territories, 2007 and 2008. Department of Environment and Climate Change, Government of the Northwest Territories.
Ref. 18. Ault, K. K., Johnson, J. E., Pinkart, H. C., and Wagner, R. S. 2012. Genetic comparison of water molds from embryos of amphibians Rana cascadae, Bufo boreas and Pseudacris regilla. Diseases of Aquatic Organisms, 99(2), 127–137.
Ref. 19. Uller, T., Sagvik, J., and Olsson, M. 2009. Pre-hatching exposure to water mold reduces size at metamorphosis in the moor frog. Oecologia, 160(1), 9–14.
Ref. 20. Robertson, E. J., Anderson, V. L., Phillips, A. J., Secombes, C. J., and van West, P. 2009. Saprolegnia—fish. Oomycete Genetics and Genomics, 407.
Ref. 21. Friend, M. 1999. Avian cholera (No. 1999-0001, pp. 75–92). US Geological Survey.
Ref. 22. Christensen, J. P., and Bisgaard, M. 2000. Fowl cholera. Revue scientifique et technique (International Office of Epizootics), 19(2), 626–637.
Ref. 23. Samuel, M. D., Botzler, R. G., and Wobeser, G. A. 2007. Avian cholera. Infectious diseases of wild birds, 239–269.
Ref. 24. Descamps, S., Jenouvrier, S., Gilchrist, H. G., and Forbes, M. R. 2012. Avian cholera, a threat to the viability of an Arctic seabird colony? PloS one, 7(2), e29659.
Ref. 25. Dick, T. A., Nelson, P. A., and Choudhury, A. 2001. Diphyllobothriasis: Update on human cases, foci, patterns and sources of human infections and future considerations. Southeast Asian Journal of Tropical Medicine and Public Health, 32, 59–76.
Ref. 26. Dupouy-Camet, J., and Peduzzi, R. 2014. Helminth-Trematode: Diphyllobothrium. Encyclopedia of Food Safety, 2014.
Ref. 27. Daschner, A. 2016. Risks and possible health effects of raw fish intake. In Fish and fish oil in health and disease prevention (pp. 341–353). Academic Press.
Ref. 28. Henriksen, E. H., Frainer, A., Knudsen, R., Kristoffersen, R., Kuris, A. M., Lafferty, K. D., and Amundsen, P. A. 2019. Fish culling reduces tapeworm burden in Arctic charr by increasing parasite mortality rather than by reducing density‐dependent transmission. Journal of Applied Ecology, 56(6), 1482–1491.
Ref. 29. Hocking D.J, Babbitt K.J. 2014. Amphibian Contributions to Ecosystem Services. Herpetological Conservation and Biology 9(1):1−17. Available at http://www.herpconbio.org/Volume_9/Issue_1/Hocking_Babbitt_2014.pdf
Ref. 30. Smith, A. M., and M. Green, D. 2005. Dispersal and the metapopulation paradigm in amphibian ecology and conservation: Are all amphibian populations metapopulations? Ecography, 28(1), 110–128.
Ref. 31. Gray, M., and Chinchar, V.G. 2015. Ranaviruses: Lethal pathogens of ectothermic vertebrates. Springer International Publishing. doi:10.1007/978-3-319-13755-1.
Ref. 32. Fisher, M. C., and Garner, T. W. 2020. Chytrid fungi and global amphibian declines. Nature Reviews Microbiology, 18(6), 332–343.
Ref. 33. OIE - World Organisation for Animal Health 2021; https://www.oie.int/en/document/ranavirusesinfection_with/
Ref. 34. Scheele, B. C., Pasmans, F., Skerratt, L. F., Berger, L., Martel, A. N., Beukema, W., ... and Canessa, S. 2019. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science, 363(6434), 1459–1463.
Ref. 35. Voyles, J., Rosenblum, E. B., and Berger, L. 2011. Interactions between Batrachochytrium dendrobatidis and its amphibian hosts: a review of pathogenesis and immunity. Microbes and Infection, 13(1), 25–32.
Ref. 36. Deguise, I., and Richardson, J. S. 2009. Prevalence of the chytrid fungus (Batrachochytrium dendrobatidis) in Western Toads in southwestern British Columbia, Canada. Northwestern Naturalist, 90(1), 35–38.
Ref. 37. D’Aoust-Messier, A. M., Echaubard, P., Billy, V., and Lesbarrères, D. 2015. Amphibian pathogens at northern latitudes: presence of chytrid fungus and ranavirus in northeastern Canada. Diseases of Aquatic Organisms, 113, 149–155.
Ref. 38. Gahl, M. K., Pauli, B. D., and Houlahan, J. E. 2011. Effects of chytrid fungus and a glyphosate‐based herbicide on survival and growth of wood frogs (Lithobates sylvaticus). Ecological Applications, 21(7), 2521–2529.
Ref. 39. Campbell L, Bower DS, Clulow S, Stockwell M, Clulow J, Mahony M. 2019. Interaction between temperature and sublethal infection with the amphibian chytrid fungus impacts a susceptible frog species. Scientific Reports 9:83. DOI:10.1038/s41598-018-35874-7
Ref. 40. Duffus, A. L., Waltzek, T. B., Stöhr, A. C., Allender, M. C., Gotesman, M., Whittington, R. J., and Marschang, R. E. 2015. Distribution and host range of ranaviruses. in Ranaviruses (pp. 9–57). Springer.
Ref. 41. Miller, D. L., Pessier, A. P., Hick, P., and Whittington, R. J. 2015. Comparative pathology of ranaviruses and diagnostic techniques. In Ranaviruses (pp. 171–208). Springer.
Ref. 42. Echaubard P, Little K, Pauli B, Lesbarrères D. 2010. Context-dependent effects of ranaviral infection on northern leopard frog life history traits. PLoS One 5:e13723
Ref. 43. Brunner, J. L., Storfer, A., Gray, M. J., and Hoverman, J. T. 2015. Ranavirus ecology and evolution: from epidemiology to extinction. In Ranaviruses (pp. 71–104). Springer.
Ref. 44. Forzán, M. J., Bienentreu, J., Schock, D. M., and Lesbarrères, D. 2019. Multi-tool diagnosis of an outbreak of ranavirosis in amphibian tadpoles in the Canadian boreal forest. Diseases of Aquatic Organisms, 135(1), 33–41.
Ref. 45. Grant, S., Bienentreu, J., Vilaca, S., Brunetti, C., Lesbarrères, D., Murray, D., and Kyle, C. 2019. Low intraspecific variation of Frog virus 3 with evidence for novel FV3-like isolates in central and northwestern Canada. Diseases of Aquatic Organisms, 134(1), 1–13.
Ref. 46. Vilaça, S., Bienentreu, J., Brunetti, C. R., Lesbarrères, D., Murray, D. L., and Kyle, C. J. 2019. Frog virus 3 genomes reveal prevalent recombination between Ranavirus lineages and their origin in Canada. Journal of Virology, JVI-00765.
Ref. 47. Bienentreu, J. F., Grayfer, L., Schock, D. M., Guerreiro, M., Mehes-Smith, M., DeWitte-Orr, S. J., and Lesbarrères, D. 2020. Sublethal effects of wild-type and a vIF-2α-knockout Frog virus 3 on postmetamorphic wood frogs (Rana sylvatica): potential for a stage-specific reservoir. FACETS, 5(1), 738–757.
Ref. 48. Romansic JM, Diez KA, Higashi EM, Johnson JE, Blaustein AR (2009) Effects of the pathogenic water mold Saprolegnia ferax on survival of amphibian larvae. Diseases of Aquatic Organisms 83:187-193
Ref. 49. Ruthig GR (2008) The influence of temperature and spatial distribution on the susceptibility of southern leopard frog eggs to disease. Oecologia 156: 895-903
Ref. 50. Ruthig GR (2009) Water molds of the genera Saprolegnia and Leptolegnia are pathogenic to the North American frogs Rana catesbeiana and Pseudacris crucifer, respectively. Diseases of Aquatic Organisms 84:173-178
Ref. 51. Working Group on General Status of NWT Species. 2021. NWT Species 2021-2025 – General Status Ranks of Wild Species in the Northwest Territories, Department of Environment and Climate Change, Government of the Northwest Territories, Yellowknife, NT. 389 pp.
Ref. 52. Tallman, R. F., Hedges, K. J., Martin, Z., Janjua, M. Y., Van Gerwen-Toyne, M., and Harris, L. N. 2015. Towards Determining Optimal Harvest Levels for Arctic Char, Salvelinus Alpinus, in Nunavut: Overview and Proposed Research Plans. Fisheries and Oceans Canada.
Ref. 53. Zhu, X., Gallagher, C.P., Howland, K.L., Harwood, L.A., and Tallman, R.F. 2017. Multimodel assessment of population production and recommendations for sustainable harvest levels of anadromous Arctic Char, Salvelinus alpinus (L.), from the Hornaday River, Northwest Territories. DFO Can. Sci. Advis. Sec. Res. Doc. 2016/116. v + 81 p.
Ref. 54. Stewart, D. B. and Bernier, L. M. J., 1999. Common parasites, diseases and injuries of freshwater fishes in the Northwest Territories and Nunavut. Zoonotics and Wildlife Disease. 30.
Ref. 55. Chiaramonte, L., Munson, D., and Trushenski, J. 2016. Climate change and considerations for fish health and fish health professionals. Fisheries, 41(7), 396–399.
Ref. 56. NERB (NWT Environmental Research Bulletin). 2019. Arctic Salmon: Community monitoring initiatives find increasing salmon in the NWT. Vol 4, Issue 17.
Ref. 57. Chinchar, V. G. 2011. Viruses infecting fish, amphibians, and reptiles. Special issue Viruses.
Ref. 58. Chai, J. Y., Murrell, K. D., and Lymbery, A. J. 2005. Fish-borne parasitic zoonoses: status and issues. International Journal for Parasitology, 35(11-12), 1233–1254.
Ref. 59. Esteban, J. G., Muñoz-Antoli, C., Borras, M., Colomina, J., and Toledo, R. 2014. Human infection by a “fish tapeworm”, Diphyllobothrium latum, in a non-endemic country. Infection, 42(1), 191–194.
Ref. 60. Bruno, D. E., and Ellis, A. E. 1996. Principles of Salmonid Culture. In Developments in Aquaculture and Fisheries Science.
Ref. 61. Van Hemert, C., Pearce, J. M., and Handel, C. M. 2014. Wildlife health in a rapidly changing North: focus on avian disease. Frontiers in Ecology and the Environment, 12(10), 548–556.
Ref. 62. Samuel, M. D., Shadduck, D. J., Goldberg, D. R., and Johnson, W. P. 2005. Avian cholera in waterfowl: the role of lesser snow and Ross's geese as disease carriers in the Playa Lakes Region. Journal of wildlife diseases, 41(1), 48–57.
Ref. 63. Gaidet, N., Cappelle, J., Takekawa, J. Y., Prosser, D. J., Iverson, S. A., Douglas, D. C., ... and Newman, S. H. 2010. Potential spread of highly pathogenic avian influenza H5N1 by wildfowl: dispersal ranges and rates determined from large‐scale satellite telemetry. Journal of Applied Ecology, 47(5), 1147–1157.
Ref. 64. Larsson, C., Comstedt, P., Olsen, B., and Bergström, S. 2007. First record of Lyme disease Borrelia in the Arctic. Vector-Borne and Zoonotic Diseases, 7(3), 453–456.
Ref. 65. Harriman, V. B., and Alisauskas, R. T. 2010. Of fleas and geese: The impact of an increasing nest ectoparasite on reproductive success. Journal of Avian Biology, 41(5), 573–579.
Ref. 66. Loiseau, C., Harrigan, R. J., Cornel, A. J., Guers, S. L., Dodge, M., Marzec, T., ... and Sehgal, R. N. 2012. First evidence and predictions of Plasmodium transmission in Alaskan bird populations. PloS one, 7(9), e44729.
Ref. 67. Cardona, C. J., Xing, Z., Sandrock, C. E., and Davis, C. E. 2009. Avian influenza in birds and mammals. Comparative immunology, microbiology and infectious diseases, 32(4), 255–273.
Ref. 68. Lai, S., Qin, Y., Cowling, B. J., Ren, X., Wardrop, N. A., Gilbert, M., ... and Yu, H. 2016. Global epidemiology of avian influenza A H5N1 virus infection in humans, 1997–2015: a systematic review of individual case data. The Lancet Infectious Diseases, 16(7), e108-e118.
Ref. 69. Samuel, M. D., Takekawa, J. Y., Samelius, G., and Goldberg, D. R. 1999. Avian cholera mortality in lesser snow geese nesting on Banks Island, Northwest Territories. Wildlife Society Bulletin, 780–787.
Ref. 70. Samuel, M. D., Shadduck, D. J., Goldberg, D. R., and Johnson, W. P. 2003. Comparison of methods to detect Pasteurella multocida in carrier waterfowl. Journal of Wildlife Diseases, 39(1), 125–135.
Ref. 71. Canadian Herpetofauna Health Working Group. 2017. Decontamination Protocol for Field Work with Amphibians and Reptiles in Canada 7pp. +ii. Available at http://www.cwhc-rcsf.ca/docs/HHWG%20Decontamination%20Protocol%202017-05...
Ref. 72. Jakob-Hoff R.M., MacDiarmid S.C., Lees C., Miller P.S., Travis D. and Kock R. 2014. Manual of Procedures for Wildlife Disease Risk Analysis. World Organisation for Animal Health, Paris, 160 pp. Published in association with the International Union for Conservation of Nature and the Species Survival Commission. Available at http://www.cbsg.org/sites/cbsg.org/files/documents/IUCN%20Wildlife%20DRA...
Ref. 73. Hartley and Sainsbury. 2017. Methods of Disease Risk Analysis in Wildlife Translocations for Conservation Purposes. Ecohealth 14(Suppl 1): 16–29.
Ref. 74. Bartholomew, J. L., and Reno, P. W. 2002. The history and dissemination of whirling disease. In American fisheries society symposium (pp. 3–24). American Fisheries Society.
Ref. 75. Sarker, S., Kallert, D. M., Hedrick, R. P., and El-Matbouli, M. 2015. Whirling disease revisited: pathogenesis, parasite biology and disease intervention. Diseases of Aquatic Organisms, 114(2), 155–175.
Ref. 76. Fetherman, E. R., Winkelman, D. L., Schisler, G. J., and Antolin, M. F. 2012. Genetic basis of differences in myxospore count between whirling disease-resistant and-susceptible strains of rainbow trout. Diseases of Aquatic Organisms, 102(2), 97–106.
Ref. 77. Barry, D. E. 2020. DNA-Based Environmental Monitoring for the Invasive Myxozoan Parasite, Myxobolus cerebralis, in Alberta, Canada. Master thesis; University of Alberta.
Ref. 78. Saleh, M., Montero, R., Kumar, G., Sudhagar, A., Friedl, A., Köllner, B., and El-Matbouli, M. 2019. Kinetics of local and systemic immune cell responses in whirling disease infection and resistance in rainbow trout. Parasites and vectors, 12(1), 1–11.
Ref. 79. Arsan, E. L., Atkinson, S. D., Hallett, S. L., Meyers, T., and Bartholomew, J. L. 2007. Expanded geographical distribution of Myxobolus cerebralis: first detections from Alaska. Journal of Fish Diseases, 30(8), 483–491.
Ref. 80. Canadian Food Inspection Agency. Government of Canada. https://inspection.canada.ca/animal-health/aquatic-animals/diseases/repo... Accessed 11 Jan 2022.
Ref. 81. Monne, I., Fusaro, A., Nelson, M. I., Bonfanti, L., Mulatti, P., Hughes, J., ... and Cattoli, G. 2014. Emergence of a highly pathogenic avian influenza virus from a low-pathogenic progenitor. Journal of virology, 88(8), 4375.
Ref. 82. MacLachlan, N. J., and Dubovi, E. J. 2017. Paramyxoviridae and pneumoviridae. Fenner’s Veterinary Virology; Elsevier: New York, NY, USA, 327–356.
Ref. 83. Rao, D. M., Chernyakhovsky, A., and Rao, V. 2009. Modeling and analysis of global epidemiology of avian influenza. Environmental Modelling and Software, 24(1), 124–134.
Ref. 84. Duignan, P. J., Van Bressem, M. F., Baker, J. D., Barbieri, M., Colegrove, K. M., De Guise, S., ... and Wellehan, J. F. 2014. Phocine distemper virus: current knowledge and future directions. Viruses, 6(12), 5093–5134.
Ref. 85. Kennedy, J. M., Earle, J. A., Omar, S., Abdullah, H. A., Nielsen, O., Roelke-Parker, M. E., and Cosby, S. L. 2019. Canine and phocine distemper viruses: Global spread and genetic basis of jumping species barriers. Viruses, 11(10), 944.
Ref. 86. Duignan, P. J., Saliki, J. T., St. Aubin, D. J., House, J. A., and Geraci, J. R. 1994. Neutralizing antibodies to phocine distemper virus in Atlantic walruses (Odobenus rosmarus rosmarus) from Arctic Canada. Journal of Wildlife Diseases, 30(1), 90–94.
Ref. 87. Nielsen, O., Stewart, R. E., Measures, L., Duignan, P., and House, C. 2000. A morbillivirus antibody survey of Atlantic walrus, narwhal and beluga in Canada. Journal of Wildlife Diseases, 36(3), 508–517.
Ref. 88. VanWormer, E., Mazet, J. A. K., Hall, A., Gill, V. A., Boveng, P. L., London, J. M., ... and Goldstein, T. 2019. Viral emergence in marine mammals in the North Pacific may be linked to Arctic sea ice reduction. Scientific reports, 9(1), 1–11.
Ref. 89. Davison, N. J., Barnett, J. E., Perrett, L. L., Dawson, C. E., Perkins, M. W., Deaville, R. C., and Jepson, P. D. 2013. Meningoencephalitis and arthritis associated with Brucella ceti in a short-beaked common dolphin (Delphinus delphis). Journal of Wildlife Diseases, 49(3), 632–636.
Ref. 90. Cvetnić, Ž., Duvnjak, S., Đuras, M., Gomerčić, T., Zdelar-Tuk, M., Reil, I., ... and Špičić, S. 2017. Brucellosis in marine mammals, with special emphasis on the Republic of Croatia. Med Sci, 44, 9–24.
Ref. 91. Hördt, A., López, M. G., Meier-Kolthoff, J. P., Schleuning, M., Weinhold, L. M., Tindall, B. J., ... and Göker, M. 2020. Analysis of 1,000+ type-strain genomes substantially improves taxonomic classification of Alphaproteobacteria. Frontiers in Microbiology, 11, 468.
Ref. 92. Nielsen, O., Nielsen, K., and Stewart, R. E. 1996. Serologie evidence of Bruceila spp. exposure in Atlantic walruses (Odobenus rosmarus rosmarus) and ringed seals (Phoca hispida) of Arctic Canada. Arctic, 383–386.
Ref. 93. Nielsen, O., Stewart, R. E., Nielsen, K., Measures, L., and Duignan, P. 2001. Serologic survey of Brucella spp. antibodies in some marine mammals of North America. Journal of Wildlife Diseases, 37(1), 89–100.
Ref. 94. Nielsen, O., Cobb, D., Stewart, R. E. A., Ryan, A., Dunn, B., Raverty, S., ... and Harwood, L. (2004, November). Results of a community-based disease monitoring program of marine mammals in arctic Canada. In Oceans' 04 MTS/IEEE Techno-Ocean'04 (IEEE Cat. No. 04CH37600) (Vol. 1, pp. 492–498). IEEE.
Ref. 95. Whatmore, A. M., Dawson, C., Muchowski, J., Perrett, L. L., Stubberfield, E., Koylass, M., ... and St. Leger, J. 2017. Characterisation of North American Brucella isolates from marine mammals. PLoS One, 12(9), e0184758.
Ref. 96. Foster, G., Osterman, B. S., Godfroid, J., Jacques, I., and Cloeckaert, A. 2007. Brucella ceti sp. nov. and Brucella pinnipedialis sp. nov. for Brucella strains with cetaceans and seals as their pReferred hosts. International Journal of Systematic and Evolutionary Microbiology, 57(11), 2688–2693.
Ref. 97. Spickler, A. R., 2018. Brucellosis in Marine Mammals. Center for Food Security and Public Health. Accessed on June 3, 2021: https://www.cfsph.iastate.edu/Factsheets/pdfs/brucellosis_marine.pdf
Ref. 98. Brenes, R., Gray, M. J., Waltzek, T. B., Wilkes, R. P., and Miller, D. L. 2014. Transmission of ranavirus between ectothermic vertebrate hosts. PloS one, 9(3), e92476.
Ref. 99. Brenes, R., Miller, D. L., Waltzek, T. B., Wilkes, R. P., Tucker, J. L., Chaney, J. C., ... and Gray, M. J. 2014. Susceptibility of fish and turtles to three ranaviruses isolated from different ectothermic vertebrate classes. Journal of Aquatic Animal Health, 26(2), 118–126.
Ref. 100. Waltzek, T. B., Miller, D. L., Gray, M. J., Drecktrah, B., Briggler, J. T., MacConnell, B., ... and Hedrick, R. P. 2014. New disease records for hatchery-reared sturgeon. I. Expansion of frog virus 3 host range into Scaphirhynchus albus. Diseases of Aquatic Organisms, 111(3), 219–227.
Ref. 101. Duignan PJ, Nielsen O, House C, Kovacs KM, Duffy N, Early G, et al. 1997. Epizootiology of morbillivirus infection in harp, hooded, and ringed seals from the Canadian Arctic and western Atlantic. J Wildl Dis 33:7-19.
